Supplement Scio Four modules 17
English
Recommended separation distances from mobile high-
frequency communication equipment
The medical device is intended for use in an electromagnetic envi-
ronment in which radiated RF disturbances are controlled. The
customer or the user of the medical device can help prevent elec-
tromagnetic interference by maintaining a minimum distance
between portable and mobile RF communications equipment
(transmitters) and the medical device as recommended below,
according to the maximum output power of the communications
equipment.
For transmitters rated at a maximum output power not listed
above, the recommended separation distance d in metres (m) can
be determined using the equation applicable to the frequency of
the transmitter, where P is the maximum output power rating of the
transmitter in watts (W) according to the transmitter manufacturer.
EMC declaration for IEC 60601-1-2, 4th edition
General information
This device was tested for electromagnetic compatibility using
accessories from the list of accessories. Other accessories may
only be used if they do not compromise the electromagnetic com-
patibility. The use of non-compliant accessories may result in
increased electromagnetic emissions or decreased electromag-
netic immunity of the device.
This device may be used in the direct vicinity of other devices only
if Dräger has approved this device arrangement. If no approval
has been given by Dräger, it must be ensured that this device func-
tions correctly in the desired arrangement before use. The instruc-
tions for use for the other devices must be followed.
Electromagnetic environment
This device may only be used in environments specified in section
"Environment of use" on page 5.
1) Field strengths from fixed transmitters, such as base stations for radio (cellular/cordless) telephones and land mobile radios, amateur radio, AM and FM
radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmit-
ters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the medical device is used exceeds the
applicable RF compliance level above, the medical device should be observed to verify normal operation. If abnormal performance is observed, additional
measures may be necessary, such as re-orienting or relocating the medical device.
2) Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
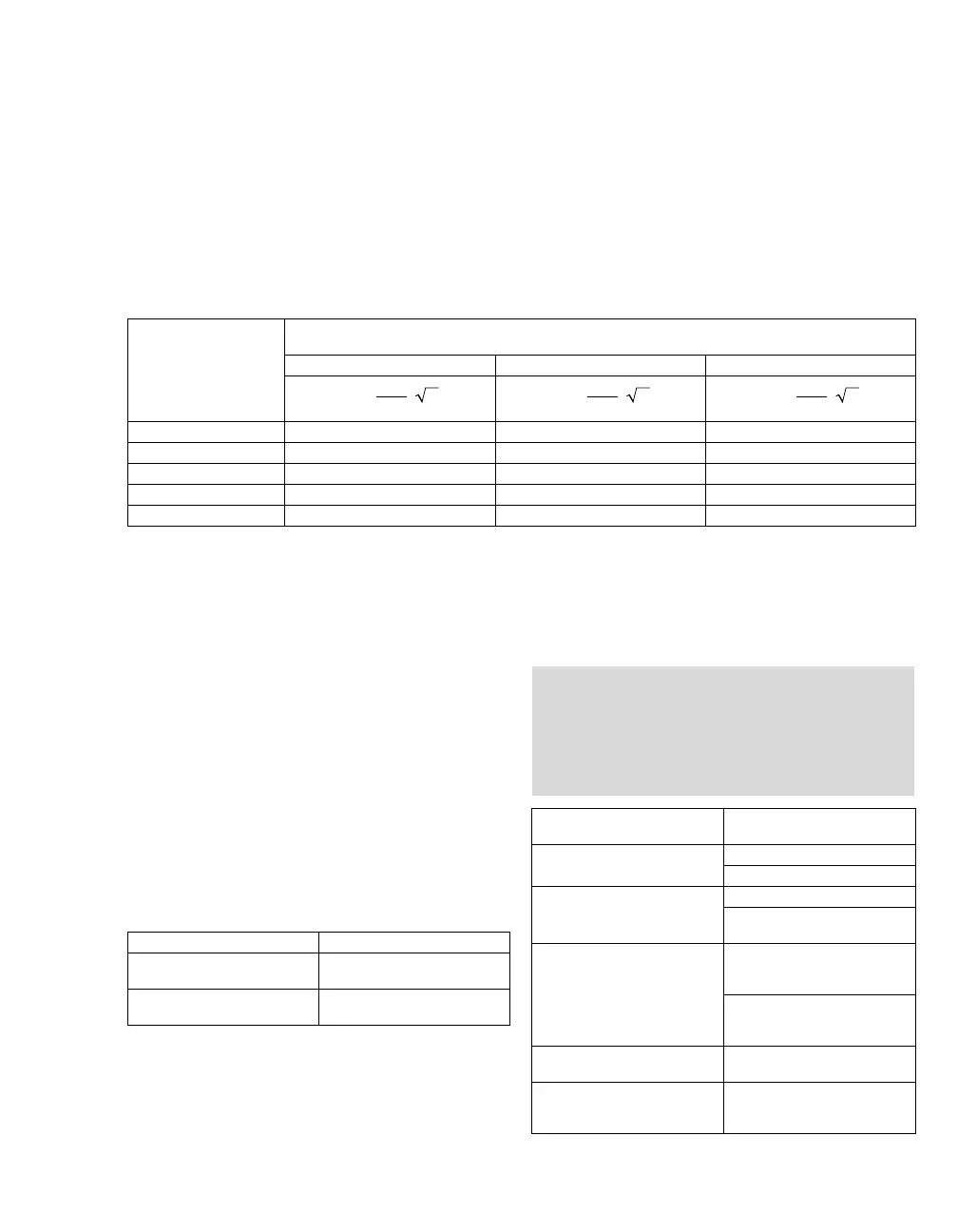
Rated maximum output
power of transmitter
W
Separation distance according to frequency of transmitter
m
150 kHz to 80 MHz 80 MHz to 800 MHz 800 MHz to 2.5 GHz
0.01 0.1 0.1 0.2
0.1 0.4 0.4 0.7
11.2 1.2 2.3
10 3.8 3.8 7.4
100121223
Emissions Compliance
Radiated emissions Class A, group 1
(30MHz to 1GHz)
Conducted emissions Class A, group 1
(150 kHz to 30 MHz)
NOTE
The emissions characteristics of this equipment make it suitable
for use in industrial areas and hospitals (CISPR 11 class A). If it
is used in a residential environment (for which CISPR 11 class
B is normally required), this equipment might not offer adequate
protection to radio-frequency communication services. The user
might need to take mitigation measures, such as relocating or
re-orienting the equipment.
Immunity against Test level and required elec-
tromagnetic environment
Electrostatic discharge (ESD)
(IEC 61000-4-2)
Contact discharge: ±8 kV
Air discharge: ±15 kV
Fast transient electrical distur-
bances (bursts)
(IEC 61000-4-4)
Power cable: ±2 kV
Longer signal input lines/out-
put lines: ±1 kV
Impulse voltages (surges)
(IEC 61000-4-5)
Voltage, external conductor –
external conductor:
±1 kV
Voltage, external conductor –
protective ground conductor:
±2 kV
Magnetic fields at mains fre-
quency (IEC 61000-4-8)
50 Hz: 30 A/m
Voltage dips and short inter-
ruptions in the supply voltage
(IEC 61000-4-11)
Voltage dips of 30 % to 100 %,
8.3 ms to 5 s, different
phase angles
 Loading...
Loading...