1-12 CASE Revision C
2060290-201
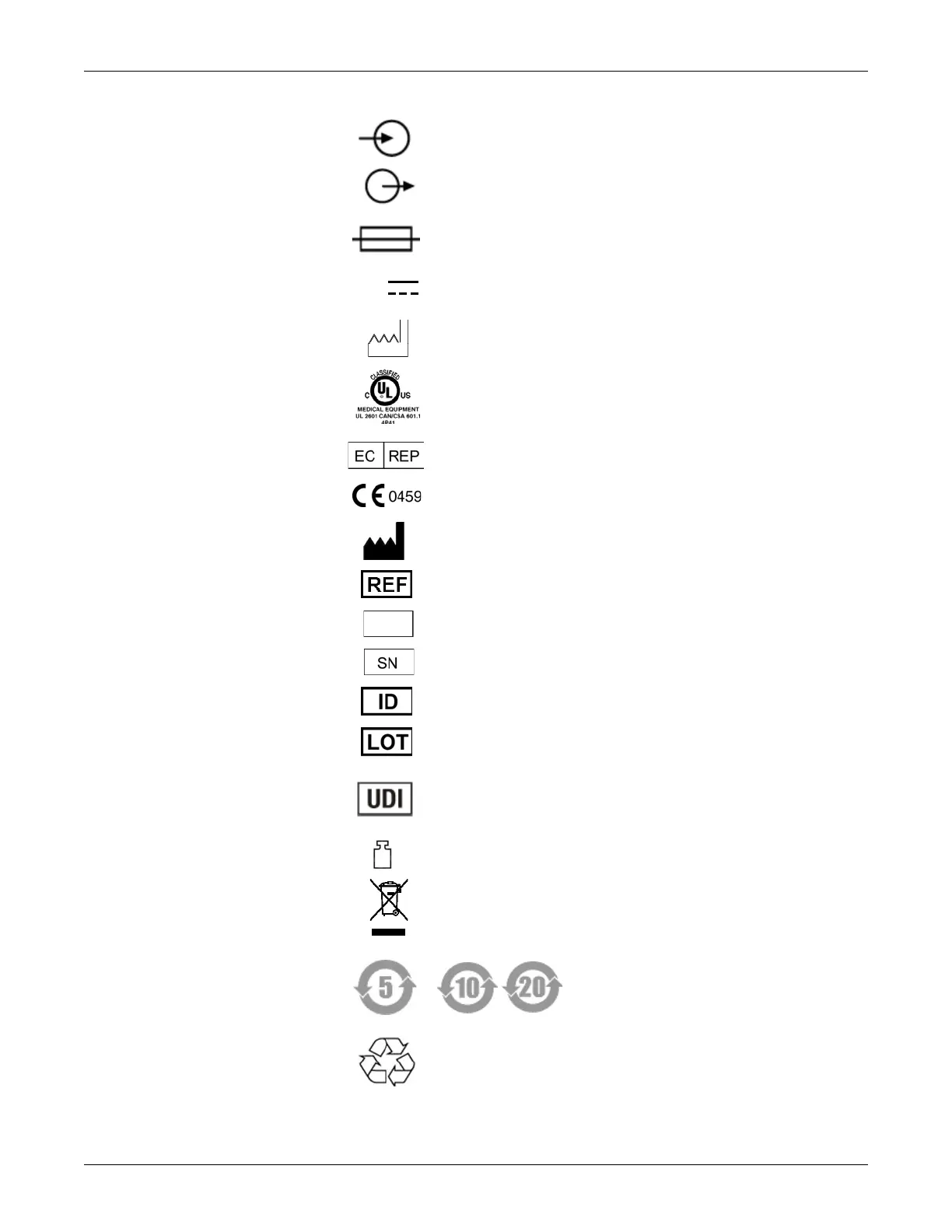
Safety Information
Signal input
Signal output
Fuse
12 V DC
The number found under this symbol is the date of manufacture in the YYYY-
MM format.
Medical Equipment — Classified with respect to electric shock, fire and
mechanical hazards only in accordance with UL 60601-1/CAN/CSA C22.2
No. 601.1, CAN/CSA C22.2 No. 601-2-25, IEC 60601-2-25, IEC 60601-1-1.
Authorized representative in the European Community.
CE marked per the Medical Device Directive 93/42/EEC of the European
Union.
Manufacturer’s identification
Order number
Revision letter
Serial number
Ident number
Lot number
Unique Device Identification is a unique marking for identification of the
medical device.
Mass
This symbol indicates that the waste of electrical and electronic equipment
must not be disposed as unsorted municipal waste and must be collected
separately. Please contact an authorized representative of the manufacturer
for information concerning the decommissioning of your equipment.
China RoHS Mobius loop symbol
REV
China RoHS pollution control label
 Loading...
Loading...