1
Introduction
Standards applied
26 / 106
VOLISTA
IFU 01781 EN 19
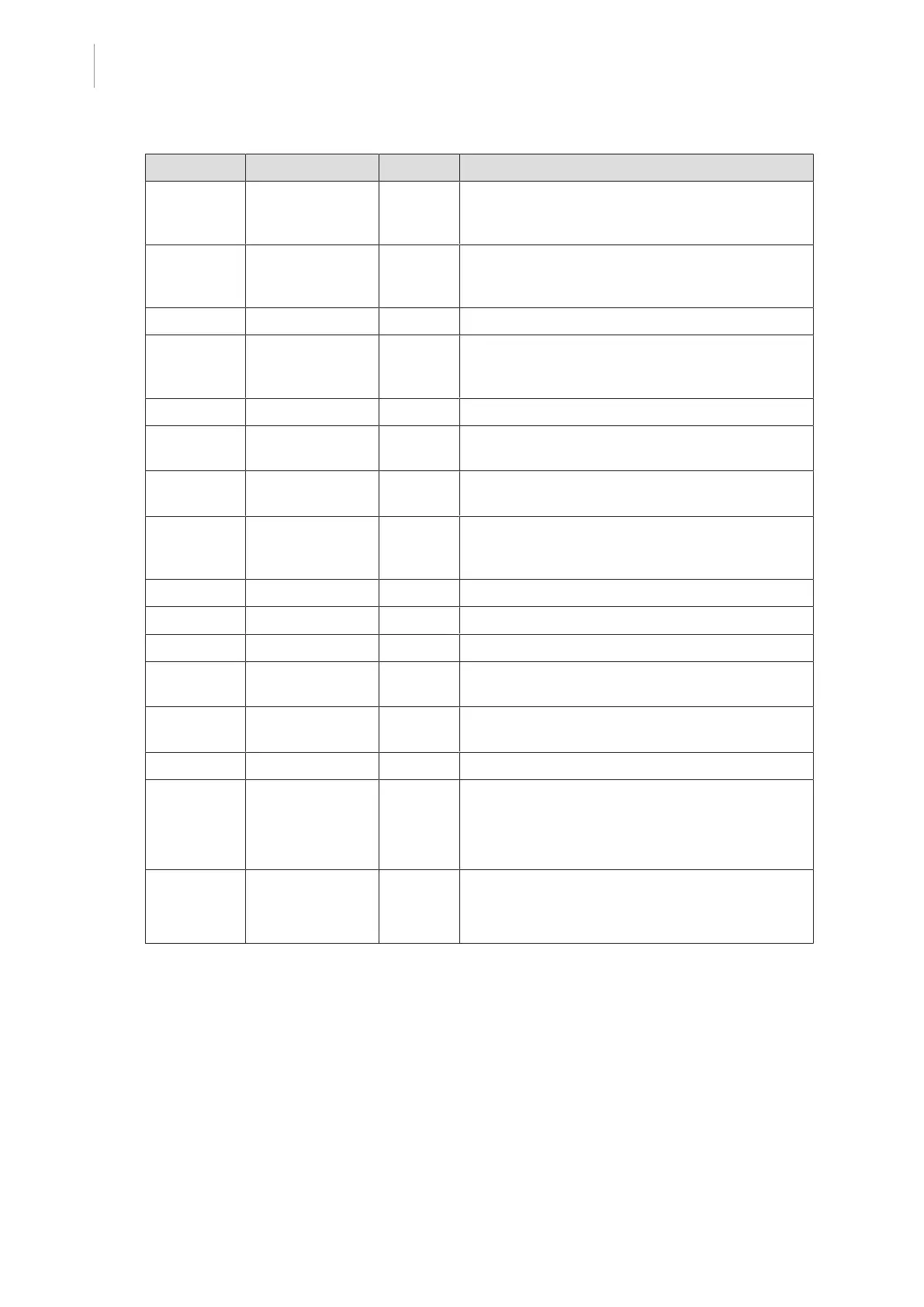
Country Reference Year Title
Argentina Dispocision
2318/2002
2002 Administración Nacional de Medicamentos, Ali-
mentos y Tecnología Médica - Registro de pro-
ductos Medicas - Reglamento
Australia TGA 236-2002 2019 Therapeutic Goods (Medical Devices) Regula-
tions 2002. Statutory Rules No. 236, 2002 made
under the Therapeutic Goods Act 1989
Brazil RDC 665/2022 2022 GMP Requirements for Medical Devices and IVDs
Brazil RDC 185/2001 2001 Technical regulation about the registration of
medical products at ANVISA, as well as its altera-
tion, revalidation, or cancellation
Canada SOR/98-282 2021 Medical Devices Regulations
China Regulation 739 2021 Regulation for the Supervision and Administration
of Medical Devices
EU Regulation
2017/745/EU
2017 Medical Devices Regulations
Japan MHLW Ordin-
ance: MO No.
169
2021 Ministerial Ordinance on Standards for Manufac-
turing Control and Quality Control for Medical
Devices and In-Vitro Diagnostics
South Korea Act 14330 2016 Medical Device Act
South Korea Decree 27209 2016 Enforcement Decree of Medical Act
South Korea Rule 1354 2017 Enforcement Rule of the Medical Act
Switzerland RS (Odim)
812.213
2020 Medical Devices Ordinance (MedDO) of 1 July
2020
Taiwan TPAA
2018-01-31
2018 Taiwanese Pharmaceutical Affairs Act
UK Act 2021 Medical Devices Regulations 2002 No.618
USA 21CFR Part 7 2017
Title 21--Food And Drugs
Chapter I--Food and Drug Administration Depart-
ment of Health and Human Services
Subchapter A -- General
PART 7 - Enforcement policy
USA 21CFR
Subchapter H
-
Title 21--Food And Drugs
Chapter I--Food and Drug Administration Depart-
ment of Health and Human Services
Subchapter H -- Medical Devices
Tab.6: Compliance with market standards
Other information (for the People's Republic of China only)
产品名称:手术无影灯
规格型号:STANDOP VOLISTA 600, STANDOP VOLISTA 400
SN 序列号:见英文标签 生产日期:见英文标签
使用期限:10 年
注册证号:国械注进 20142015956
产品技术要求编号:国械注进 20142015956
注册人/生产企业名称:Maquet SAS 迈柯唯股份有限公司
注册人/生产企业住所:Parc de Limère Avenue de la Pomme de Pin CS 10008 Ardon 45074 Orléans Cedex 2- FRANCE
生产地址:Parc de Limère Avenue de la Pomme de Pin CS 10008 Ardon 45074 Orléans Cedex 2- FRANCE
注册人/生产企业联系方式:+33 (0) 2 38 25 88 88
代理人:迈柯唯(上海)医疗设备有限公司
代理人住所:中国(上海)自由贸易试验区美盛路 56 号 2 层 227 室
代理人电话:800 820 0207
其他内容详见说明书
 Loading...
Loading...