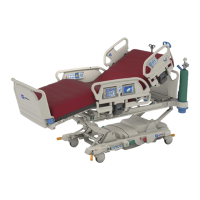
Safety
7990087_030_03 – 2080492 – 2023-01-19 19
[G] – PATENTS/PATENT hillrom.com/patents
May be subject to one or more patents. See website address
above. Hillrom companies are the holders of European, US and
other patents, as well as pending patent applications.
[H] Not available –
[I] Device label for the individual component
[J] Device label of the surgical light system
Manufacturer
Unique device identification (UDI), comprising:
– Data Matrix Code
– (01) Global Trade Item Number (GTIN)
– (11) Date of manufacture (Year Month Day)
– (21) Serial number
– (240) Part number
Baxter part number
Serial number
Medical product
The device conforms to Regulation 2017/745/EU concerning
medical devices.
Caution! Follow the warnings in the instructions for use!
The product must be disposed of at a suitable disposal facility
for the recycling of electrical and electronic devices in
accordance with the requirements of Directive
WEEE II 2012/19/EU and country-specific regulations.
variable Radio license
For detailed information, refer to Document 7990103 (Radio
Information).
Date of manufacture
No. Information
notice
Meaning

 Loading...
Loading...