Functional principles
M4 TORNADO
19
3 Functional principles
This section offers a short general description of the functional principles of the M4 TORNADO.
For a more comprehensive description, please refer to the reference manual for Physical
Principles of Micro-XRF.
3.1 X-ray Fluorescence
The basis of X-ray fluorescence analysis is an excitation and relaxation process within the atomic
electron shell. In atom physics, fluorescence is generally defined as a two-stage process in which
an atom passes into an excited status by an external energy transfer and then passes back into
the initial state on spontaneous emission of a photon.
One characteristic of X-ray fluorescence in comparison to optical fluorescence is that the
excitation occurs with distinctly higher energies and that the emitted fluorescence radiation lies
within the X-ray range. Because inner atom shells are involved this process is predominantly
specific to elements and not specific to molecules.
X-ray fluorescence analysis is a method for the determination of the element content in samples.
Conclusions about the chemical bonds cannot be made directly this way.
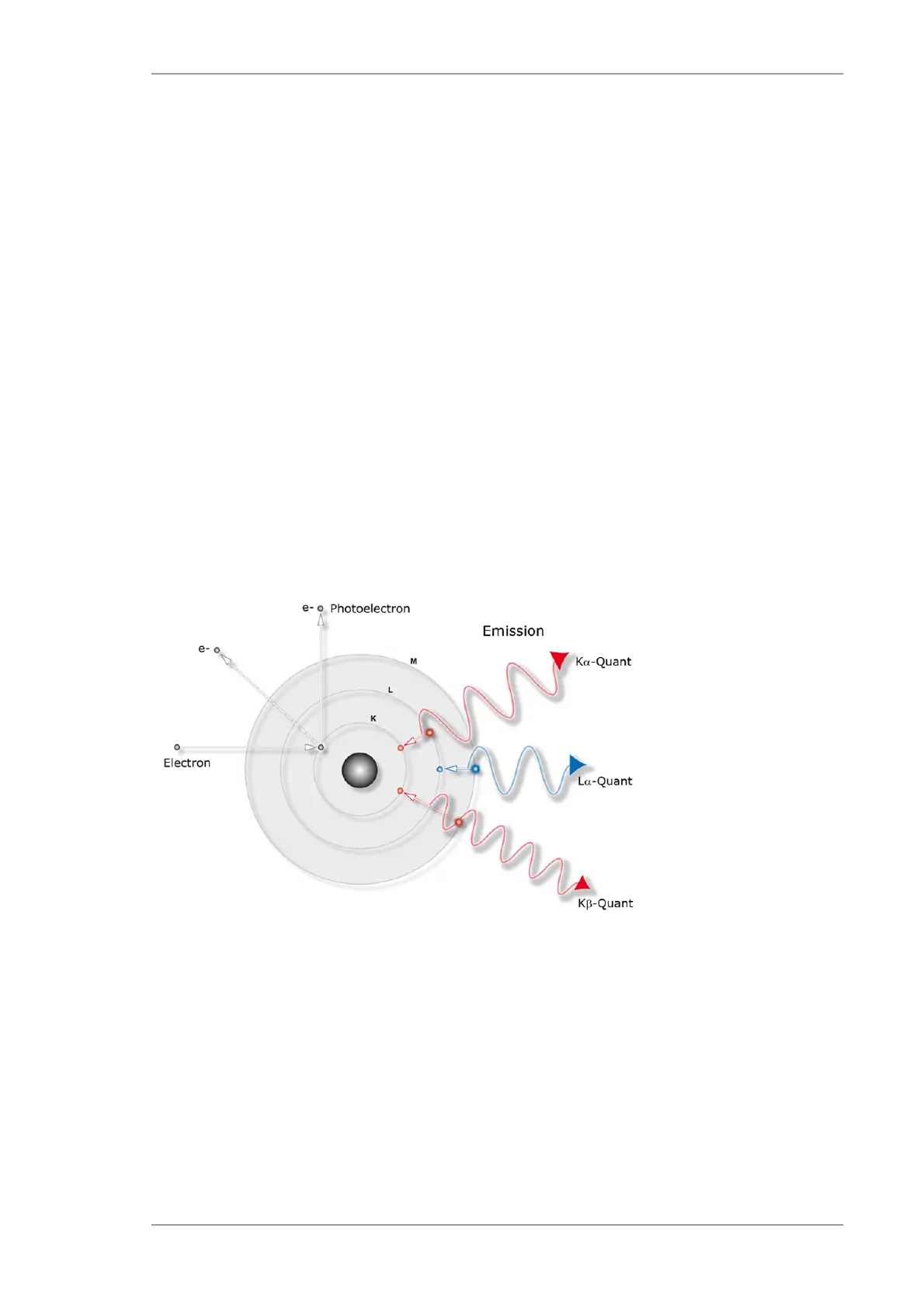
1. ionization of an
inner shell by an
X-ray photon
2. refilling of the gap
with an electron from
a higher energy level
3. emission of an
X-ray photon
Fig. 2 Emergence of X-ray fluorescence radiation
Fig. 2 illustrates the process schematically. A high-energetic electron, proton, or X-ray photon
ionizes an inner energy level, e.g. the K-shell of an atom. After the ionization, a refilling of the gap
by an electron from a higher energy level occurs rapidly. The energy difference between the two
states is emitted as an X-ray photon. This radiation is called X-ray fluorescence.
This instrument description just deals with X-ray fluorescence, which is excited by means of X-ray
radiation. Important rules of X-ray fluorescence are:
The possible excitation states and shell transitions are subject to the laws of atomic physics
and result in a line spectrum, which is characteristic for each element.

 Loading...
Loading...