Control Testing and Quality Control
146
If you use a new test strip lot and have not
inserted the code chip yet, you must do so
now. Otherwise you cannot perform a quality
control test.
As with the test strips, a code chip is also pro-
vided with the control materials. The informa-
tion on the code chip is stored in the memory
so you can use the same control materials
again at any time.
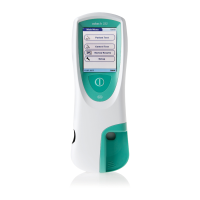
8 Select the code stored for your current
control material, or touch New to use a
new control material.
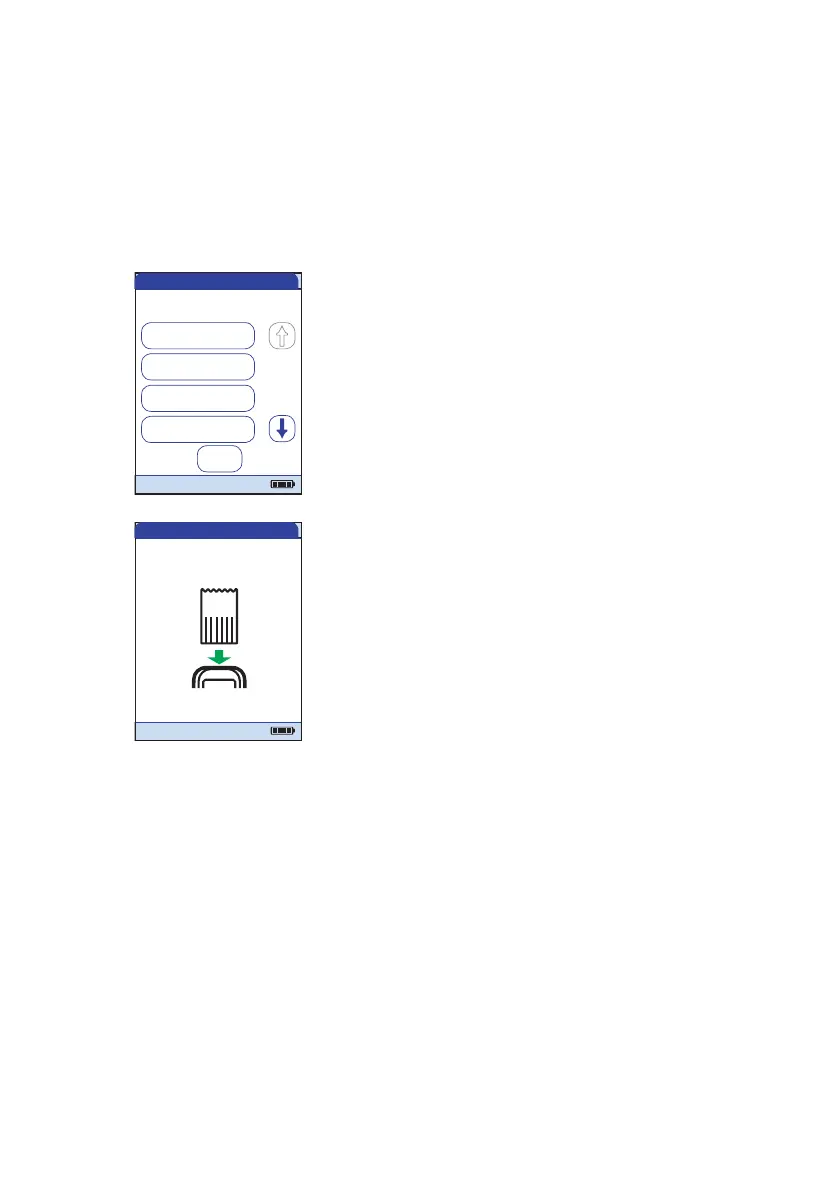
If you are using a new control material,
remove the code chip from the meter and
insert the code chip that came with the
control material instead.
QC Test - Sel. QC Lot
pro BNP PB1234
JONES,TOM
Par:
Op:
Code:
New
Code PBC014
Code PBC016
Code PBC017
Code PBC018
04/19/2016
QC Test - Ins. Chip
Insert Code
Chip
PBC014
proBNP PB1234
PBC014
JONES, TOM
Par:
Op:
Code:
QC:
04/19/2016
QC
 Loading...
Loading...