ThinPrep™ 5000 System Instructions for Use English AW-22289-001 Rev. 003 11-2021 8/36
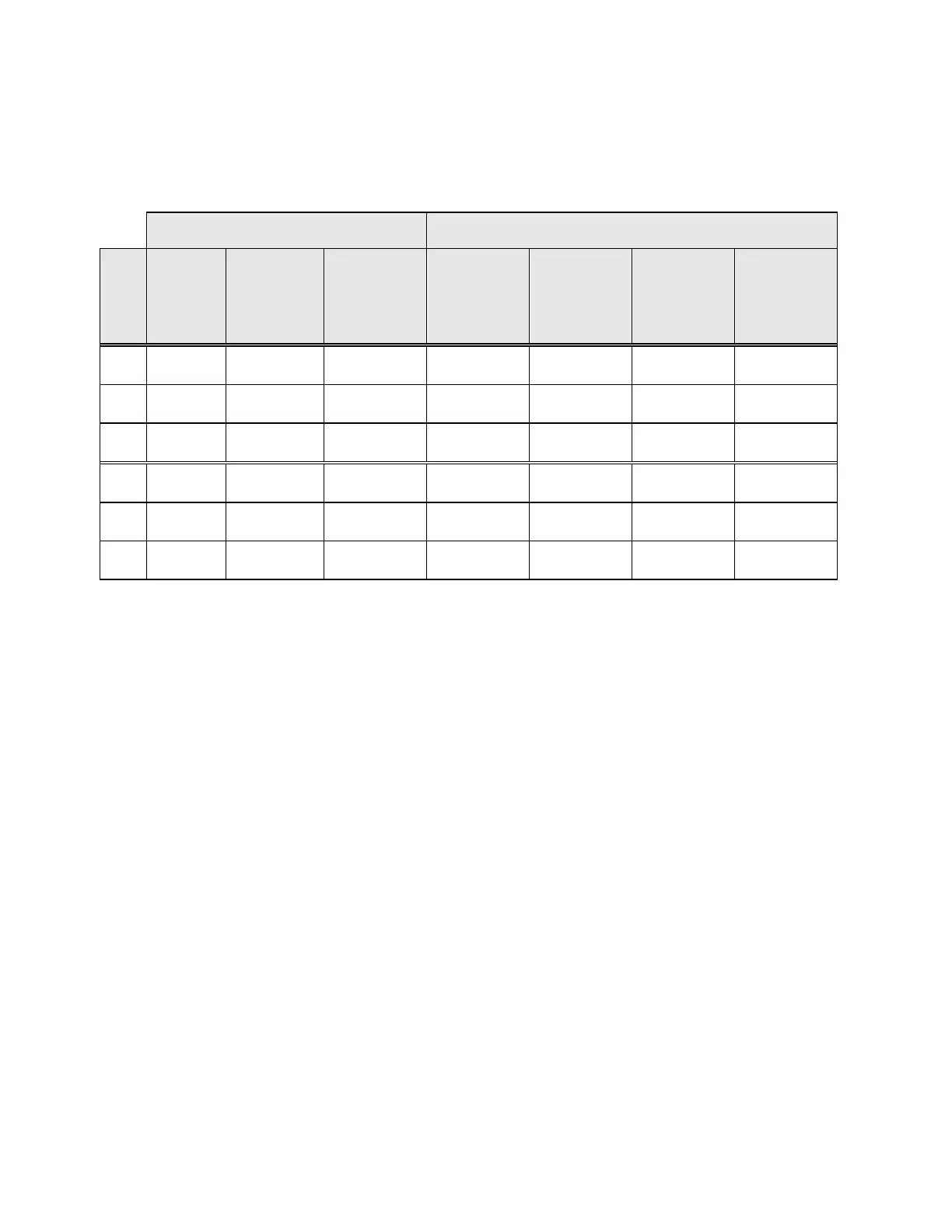
Table 1 describes the laboratories and the patient populations.
Table 1: Site Characteristics
Laboratory Characteristics Clinical Study Demographics
Site Type of
Patient
Population
Laboratory
Volume -
Smears per
Year
Cases Patient
Age Range
Post-
Meno-pausal
Previous
Abnormal Pap
Smear
Convent.
Prevalence
LSIL+
S1 Screening 300,000 1,386 18.0 - 84.0 10.6% 8.8% 2.3%
S2 Screening 100,000 1,668 18.0 - 60.6 0.3% 10.7% 2.9%
S3 Screening 96,000 1,093 18.0 - 48.8 0.0% 7.1% 3.8%
H1 Hospital 35,000 1,046 18.1 - 89.1 8.1% 40.4% 9.9%
H2 Hospital 40,000 1,049 18.1 - 84.4 2.1% 18.2% 12.9%
H3 Hospital 37,000 981 18.2 - 78.8 11.1% 38.2% 24.2%
Clinical Study Results
The diagnostic categories of The Bethesda System were used as the basis of the comparison
between conventional and ThinPrep™ findings from the clinical study. The diagnostic
classification data and statistical analyses for all clinical sites are presented in Tables 2 through 11.
Cases with incorrect paperwork, patient’s age less than 18 years, cytologically unsatisfactory
slides, or patients with a hysterectomy were excluded from this analysis. Few cases of cervical
cancer (0.02%
3
) were represented in the clinical study, as is typical in the United States patient
population.
 Loading...
Loading...