ThinPrep™ 5000 System Instructions for Use English AW-22289-001 Rev. 003 11-2021 15/36
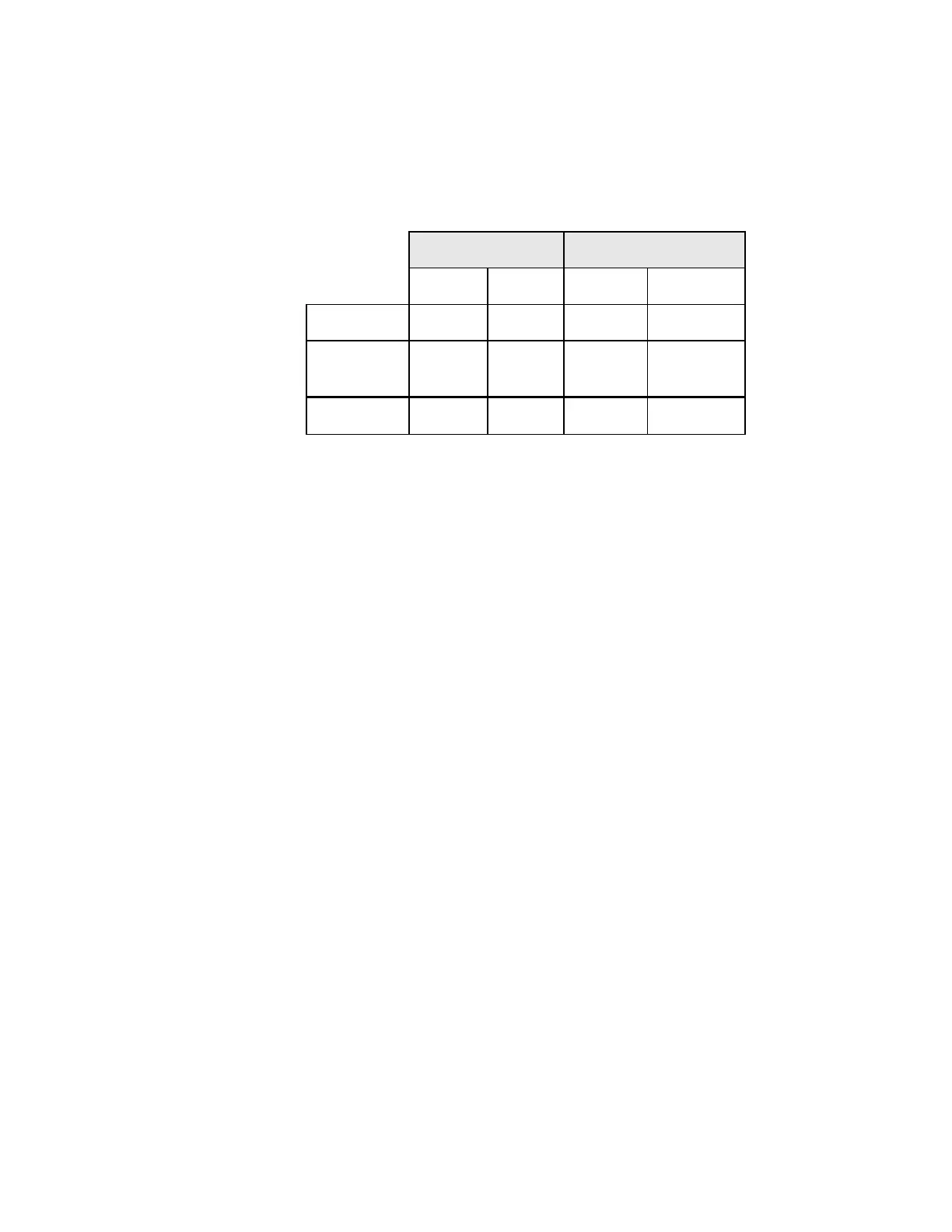
Table 11 shows the rates of detection for infection, reactive changes, and the total benign cellular
changes for both the ThinPrep™ and conventional methods at all sites.
Table 11: Benign Cellular Changes Results
ThinPrep Conventional
N % N %
Benign
Cellular
Changes
Infection
1392 20.6 1348 20.0
Reactive
Changes
412 6.1 471 7.0
Total*
1592 23.6 1591 23.6
* Total includes some patients that may have had both an infection and reactive cellular change.
Tables 12, 13, and 14 show the specimen adequacy results for the ThinPrep method and
conventional smear method for all of the study sites. Of the 7,360 total patients enrolled, 7,223
are included in this analysis. Cases with patient’s age less than 18 years or patients with a
hysterectomy were excluded from this analysis.
Two additional clinical studies were conducted to evaluate specimen adequacy results when
samples were deposited directly into the PreservCyt™ vial, without first making a conventional
Pap smear. This specimen collection technique is the intended use for the ThinPrep 2000
System. Tables 15 and 16 present the split sample and direct to vial results.
 Loading...
Loading...