Installation – ZP Oxygen Sensor System Overview 2-31
FlexFit – Linkageless Control – Revision 1.0
PREFERRED
UTILITIES MFG CORPORATION
II
NN
SS
TT
AA
LL
LL
AA
TT
II
OO
NN
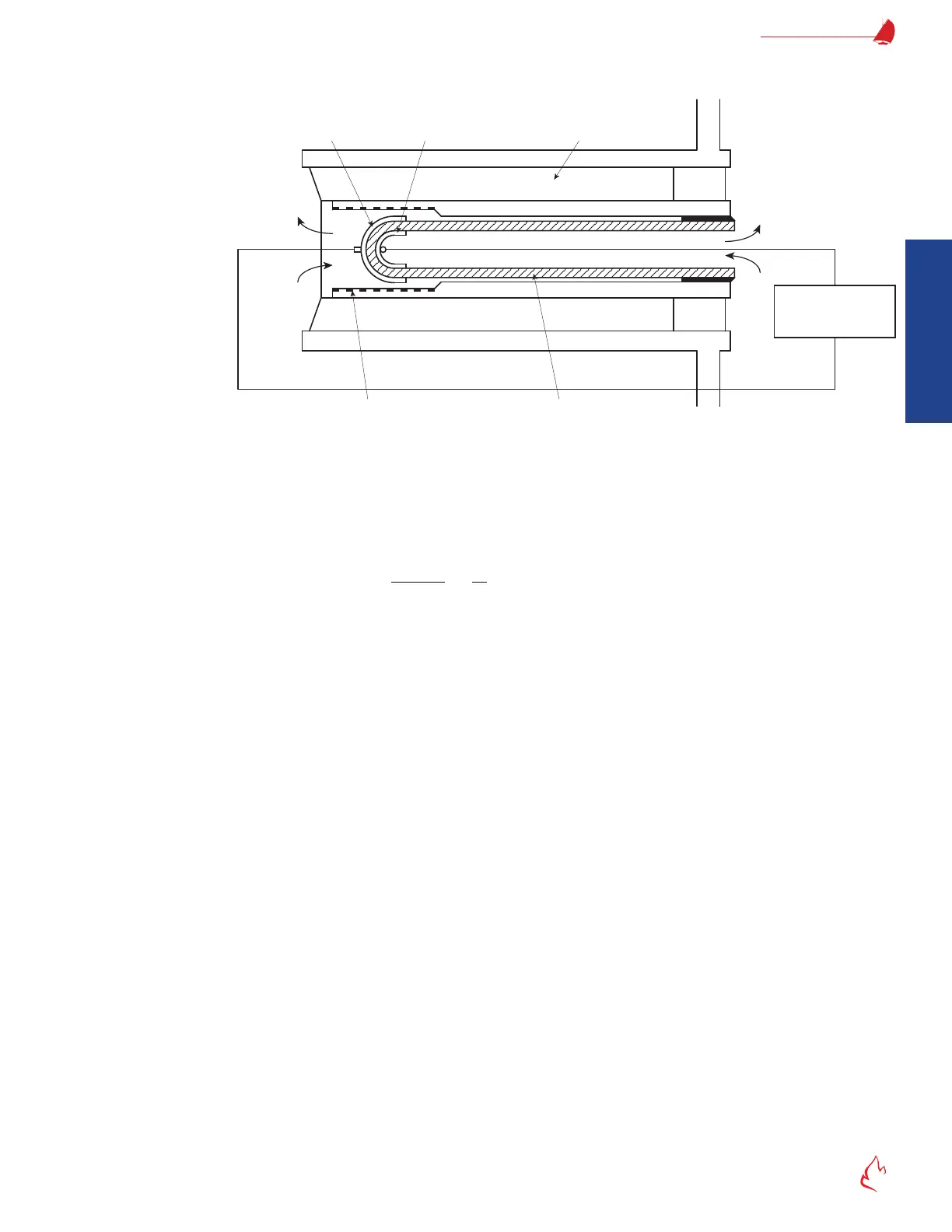
cell. Calibration gas can be injected into space behind the ceramic and quartz lters to allow on-line calibration without removal from
the stack.
Figure 2 – 21
Measuring
Electrode
Reference
Electrode
Thermal
Insulator
Zirconia
Ceramic
Electromotive
Force
Air
Detector Measuring Principle
Zirconia (ZrO
2
) ceramic sintered with a small amount of yttrium (Y
2
O
3
) is a solid electrolyte with oxygen ion conductivity at
temperatures above 500 °C. A solid electrolyte tube, coated with porous platinum on both surfaces, acts as an oxygen sensor. The
differential oxygen concentration (% ue gas oxygen vs. % room air oxygen), in contact with both platinum electrodes, produces a
voltage, related to the Nernst equation, as follows:
Figure 2 – 22
1
2
P
C R T
E ln
4F P
× ×
=
( )
1 2
E Voltage Output Signal C Calibration Coefficient
R Gas Constant T Temperature of Electrodes K
F Faraday Constant P Re ference Air O Concentration
= =
= = °
= =
2 2
P Measured O Concentration=
Nernst Equation
A ceramic heater with type R thermocouple temperature feedback is used to maintain the zirconia oxygen sensor at precisely
800 °C (1472 °F). The FlexFit monitors the thermocouple and regulates the power applied to the heater.
Ambient air provides the oxygen for the reference side of the cell. Air circulates around the reference electrode of the zirconia
element. The air is rapidly circulated by convection because the sensor is very hot and the gas volume is small. The sample gas
reaches the measuring electrode by rapid convection in a manner like the reference electrode.
When troubleshooting a zirconium oxide oxygen analyzer, it is important to remember that the cell makes a differential measure-
ment. Said another way, the cell compares the unknown oxygen percentage in the ue gas against the known oxygen percentage
in the ambient air inside the cell. Ambient air typically is 20.6%; however, it can range from 19.5% to 20.9% as relative humidity and
temperature change.
If the ue gas duct is pressurized, and a duct leaks allow ue gas to enter the detector head, the ambient oxygen percentage
can be substantially lower. Combustible gases in the ambient air will consume the oxygen on the surface of the cell and will lower
the percent oxygen in the ambient air inside the cell.
If the ambient oxygen percentage is low, a zirconia cell will sense a lower differential which will cause the analyzer to indicate
a higher oxygen level than is truly in the ue gas.
For typical mV versus % O
2
values for a new detector, see "Table 5 – 3 Cell mV Vs. % Oxygen" on page 5-89. As the detector
ages, the mV values gradually get smaller. All ZrO
2
sensors should be checked and/or re-calibrated 1-2 times per year with certied
calibration gases.
 Loading...
Loading...