Appendix A: Specifications
he EV1000 Clinical Platform NI measures blood
pressure and Cardiac Output (CO) when used with the
Heart Reference Sensor, Pressure Controller and Clear-
Sight Finger Cuff(s).
Appendix A includes summaries of the following:
• Physical and Mechanical Specifications
• Environmental Specifications
• Base Parameter Specifications
• Accessories for use with the EV1000 Clinical Platform NI
• Technical Specifications
Table A-1 Physical and Mechanical Specifications
Monitor
Weight 2.1 kg (4.6 lbs)
Dimensions Height 226 mm
Width 296 mm
Depth 58.6 mm
Display Active Area 257 mm (10.4”)
Resolution 800 x 600 LCD
Operating System Windows XPe
Speaker count 2
Monitor (Advantech Model)
Weight 2 kg (4.4 lbs)
Dimensions Height 217 mm
Width 280 mm
Depth 46 mm
Display Active Area 266 mm (10.4”)
Resolution 1024 x 768 LCD
Operating System Windows XPe
Speaker count 2
Pump-Unit
Weight (with Bracket) 3.1 kg (6.8 lbs)
Dimensions
(with Bracket)
Height 280 mm
Width 195 mm
Depth 165 mm
Pressure Controller
Weight 0.35 kg
(0.77 lbs)
Dimensions Height 97 mm
Width 54 mm
Depth 35 mm
Cable Length 3.5 m
Heart Reference Sensor
Weight 36 g
(0.08 lbs)
Dimensions Length 1.2 m
ClearSight Finger Cuff
Maximum Weight 11g
(0.02 lbs)
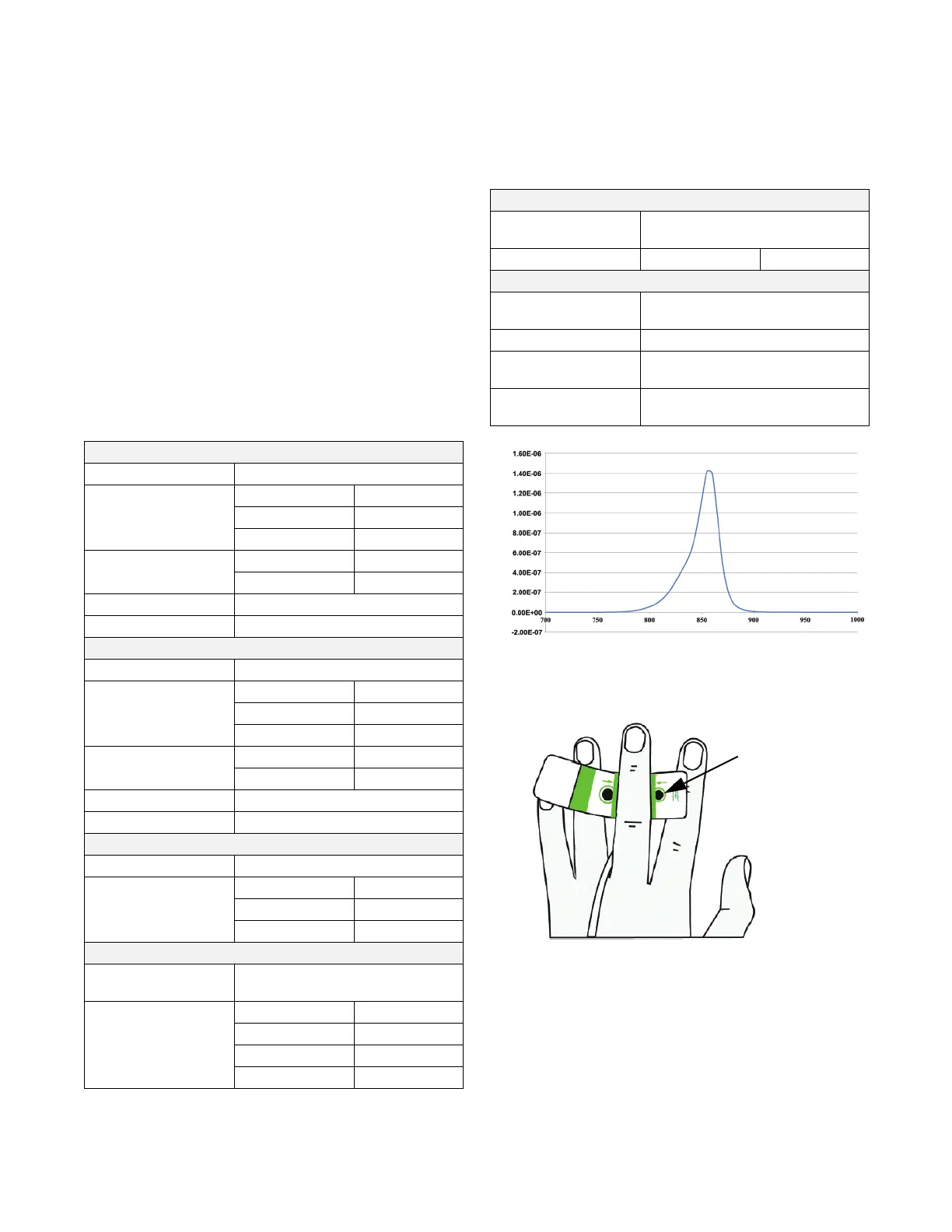
LED Spectral Irradiance See Figure A-1
Max Optical Output
over treatment area
0.013 mWatts
Max variation of output
over treatment area
50%
Figure A-1 Spectral Irradiance
Figure A-2 Location of Light Emission
Aperture
Table A-1 Physical and Mechanical Specifications
Wavelength (nm
Irradiance (Watt/cm
2
)

 Loading...
Loading...