ThinPrep™ 5000 System Instructions for Use English AW-22289-001 Rev. 003 11-2021 18/36
Direct-to-vial Endocervical Component (ECC) Studies
For the intended use of the ThinPrep™ 2000 System, the cervical sampling device will be rinsed
directly into a PreservCyt™ vial, rather than splitting the cellular sample. It was expected that this
would result in an increase in the pick-up of endocervical cells and metaplastic cells. To verify
this hypothesis, two studies were performed using the direct-to-vial method and are summarized
in Table 16. Overall, no difference was found between ThinPrep and conventional methods in
these two studies.
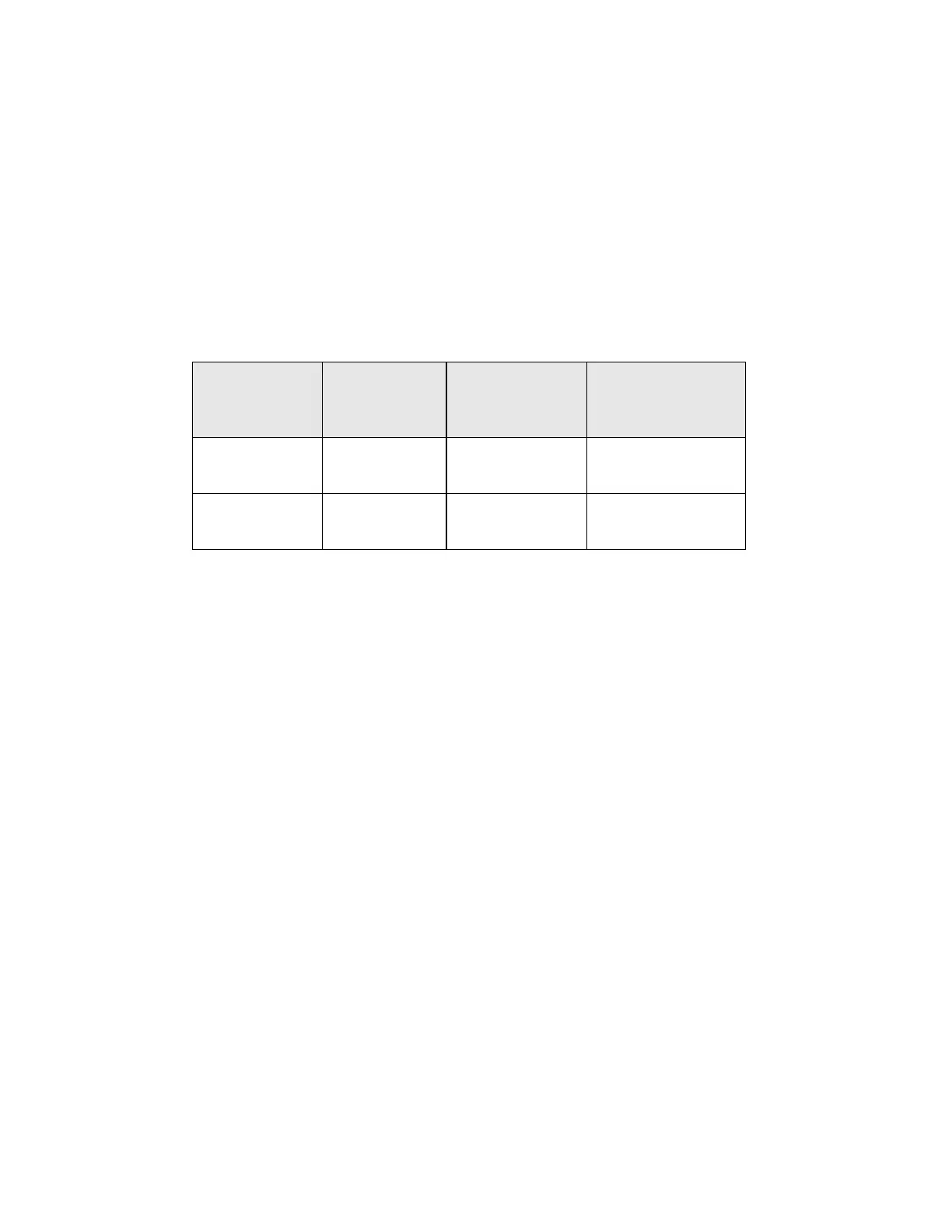
Table 16: Summary of Direct-to-vial Endocervical Component (ECC) Studies
Study
Number of
Evaluable
Patients
SBLB due to No
Endocervical
Component
Comparable
Conventional Pap
Smear Percentage
Direct-to-Vial
Feasibility
299 9.36% 9.43%
1
Direct-to-Vial
Clinical Study
484 4.96% 4.38%
2
1. Direct-to-Vial Feasibility study compared to overall clinical investigation conventional Pap
smear SBLB-No Endocervical Component rate.
2. Direct-to-Vial Clinical study compared to site S2 clinical investigation conventional Pap
smear SBLB-No Endocervical Component rate.
Direct-to-Vial HSIL+ Study
Following initial FDA approval of the ThinPrep System, Hologic conducted a multi-site direct-to-
vial clinical study to evaluate the ThinPrep 2000 System versus conventional Pap smear for the
detection of High Grade Squamous Intraepithelial and more severe lesions (HSIL+). Two types of
patient groups were enrolled in the trial from ten (10) leading academic hospitals in major
metropolitan areas throughout the United States. From each site, one group consisted of
patients representative of a routine Pap test screening population and the other group made up
of patients representative of a referral population enrolled at the time of colposcopic
examination. The ThinPrep specimens were collected prospectively and compared against a
historical control cohort. The historical cohort consisted of data collected from the same clinics
and clinicians (if available) used to collect the ThinPrep specimens. These data were collected
sequentially from patients seen immediately prior to the initiation of the study.
The results from this study showed a detection rate of 511 / 20,917 for the conventional Pap
smear versus 399 / 10,226 for the ThinPrep slides. For these clinical sites and these study
populations, this indicates a 59.7% increase in detection of HSIL+ lesions for the ThinPrep
specimens. These results are summarized in Table 17.
 Loading...
Loading...