ThinPrep™ 5000 System Instructions for Use English AW-22289-001 Rev. 003 11-2021 19/36
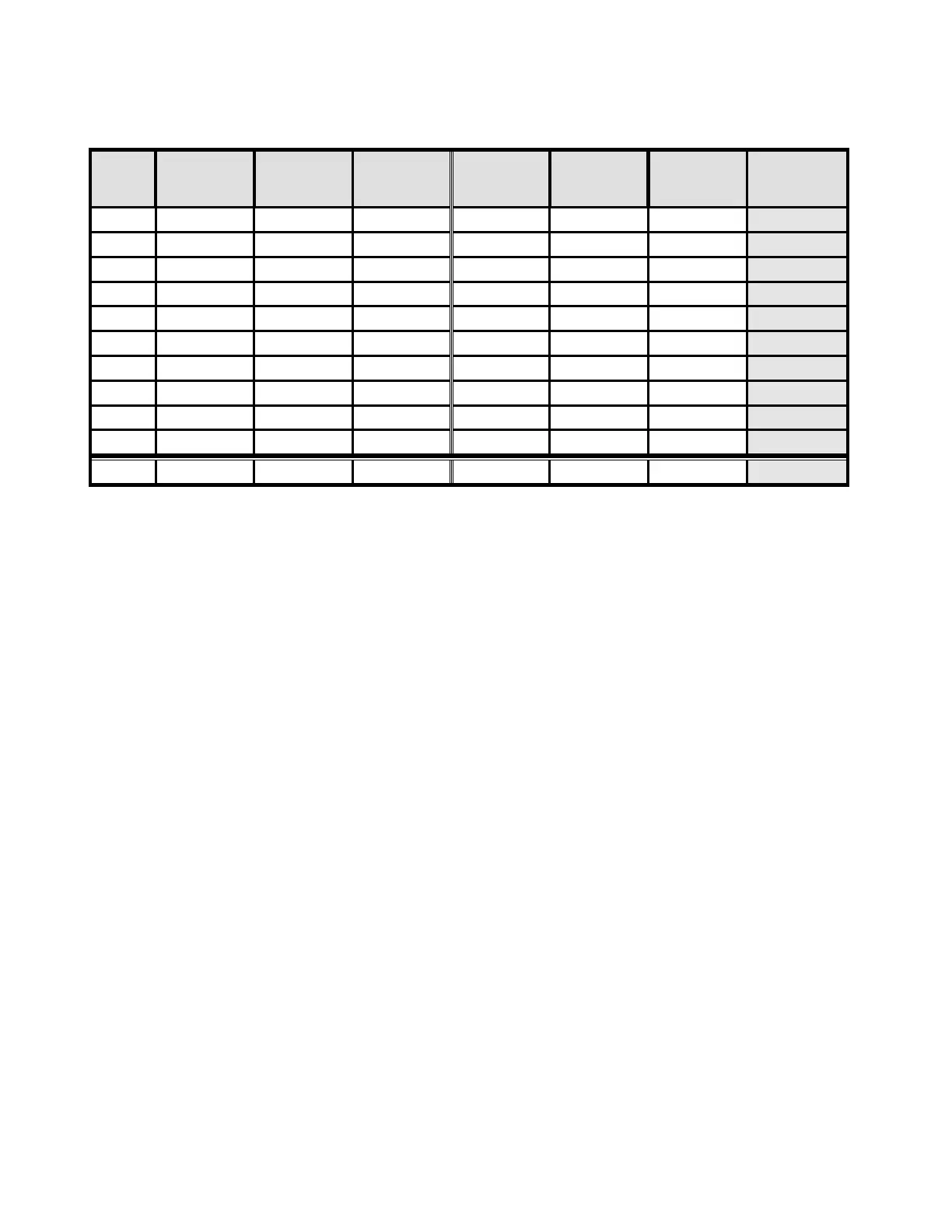
Table 17: Summary of Direct-to-Vial HSIL+ Study
Site Total CP (n)
HSIL+ Percent (%) Total TP (n) HSIL+ Percent (%)
Percent
Change (%)
S1
2,439 51 2.1
1,218
26 2.1 +2.1
S2 2,075 44 2.1
1,001
57 5.7 +168.5
S3 2,034 7 0.3
1,016
16 1.6 +357.6
S4 2,043 14 0.7
1,000
19 1.9 +177.3
S5 2,040 166 8.1
1,004
98 9.8 +20.0
S6 2,011 37 1.8
1,004
39 3.9 +111.1
S7 2,221 58 2.6
1,000
45 4.5 +72.3
S8 2,039 61 3.0
983
44 4.5 +49.6
S9 2,000 4 0.2
1,000
5 0.5 +150.0
S10 2,015 69 3.4
1,000
50 5.0 +46.0
Total 20,917 511 2.4 10,226 399 3.9 59.7( p<0.001)
Percent Change (%) = ((TP HSIL+/TP Total)/(CP HSIL+/CP Total)-1) *100
Glandular Disease Detection – Published Studies
The detection of endocervical glandular lesions is an essential function of the Pap test. However,
abnormal glandular cells in the Pap sample may also originate from the endometrium or from
extrauterine sites. The Pap test is not intended to be a screening test for such lesions.
When suspected glandular abnormalities are identified, their accurate classification as true
glandular versus squamous lesions is important for proper evaluation and subsequent treatment
(e.g. choice of excisional biopsy method versus conservative follow-up). Multiple peer-reviewed
publications
4-9
report on the improved ability of the ThinPrep 2000 System to detect glandular
disease versus the conventional Pap smear. Although these studies do not consistently address
sensitivity of different Pap testing methods in detecting specific types of glandular disease, the
reported results are consistent with more frequent biopsy confirmation of abnormal glandular
findings by the ThinPrep Pap Test compared to conventional cytology.
Thus, the finding of a glandular abnormality on a ThinPrep Pap Test slide merits increased
attention for definitive evaluation of potential endocervical or endometrial pathology.
ThinPrep 5000 Processor Compared to ThinPrep 2000 System
A study was conducted to estimate the Positive Percent Agreement (PPA) and Negative Percent
Agreement (NPA) for specimens processed on the ThinPrep 5000 processor as compared with
processing using the ThinPrep 2000 System.
 Loading...
Loading...