ThinPrep™ 5000 System Instructions for Use English AW-22289-001 Rev. 003 11-2021 20/36
Clinical Study Design
The study was a prospective, multi-center, split-sample, blinded evaluation of ThinPrep slides
of
known
diagnoses generated from residual cytological specimens. The study was conducted at
Hologic, Inc., Marlborough, MA and at two external laboratories in the United States.
One thousand two hundred sixty (1260) specimens were procured for and selected from
Hologic’s Residual Specimen Inventory for Hologic’s laboratory. At the external study sites
specimens were from residual cytological specimens from the clinical laboratory (after the
laboratory has prepared a slide from the vial and has signed-out the case per standard practice).
The laboratory’s specimens were only supplemented from Hologic’s inventory with the rarest
cytologic diagnostic categories (AGUS and Cancer), if needed. Slides prepared for the study were
from specimens processed within 6 weeks of specimen collection.
All study specimens were processed both on a ThinPrep 5000 processor and a ThinPrep 2000
system. The order in which the slides were processed was alternated in blocks of 20. All slides
were stained, coverslipped, and read manually following standard laboratory procedures; all
slides prepared at a site were reviewed independently by each of the three (3) pairs of
cytotechnologists/pathologists. All cytologic diagnoses were determined in accordance with the
Bethesda System 2001 criteria for all slides
1
.
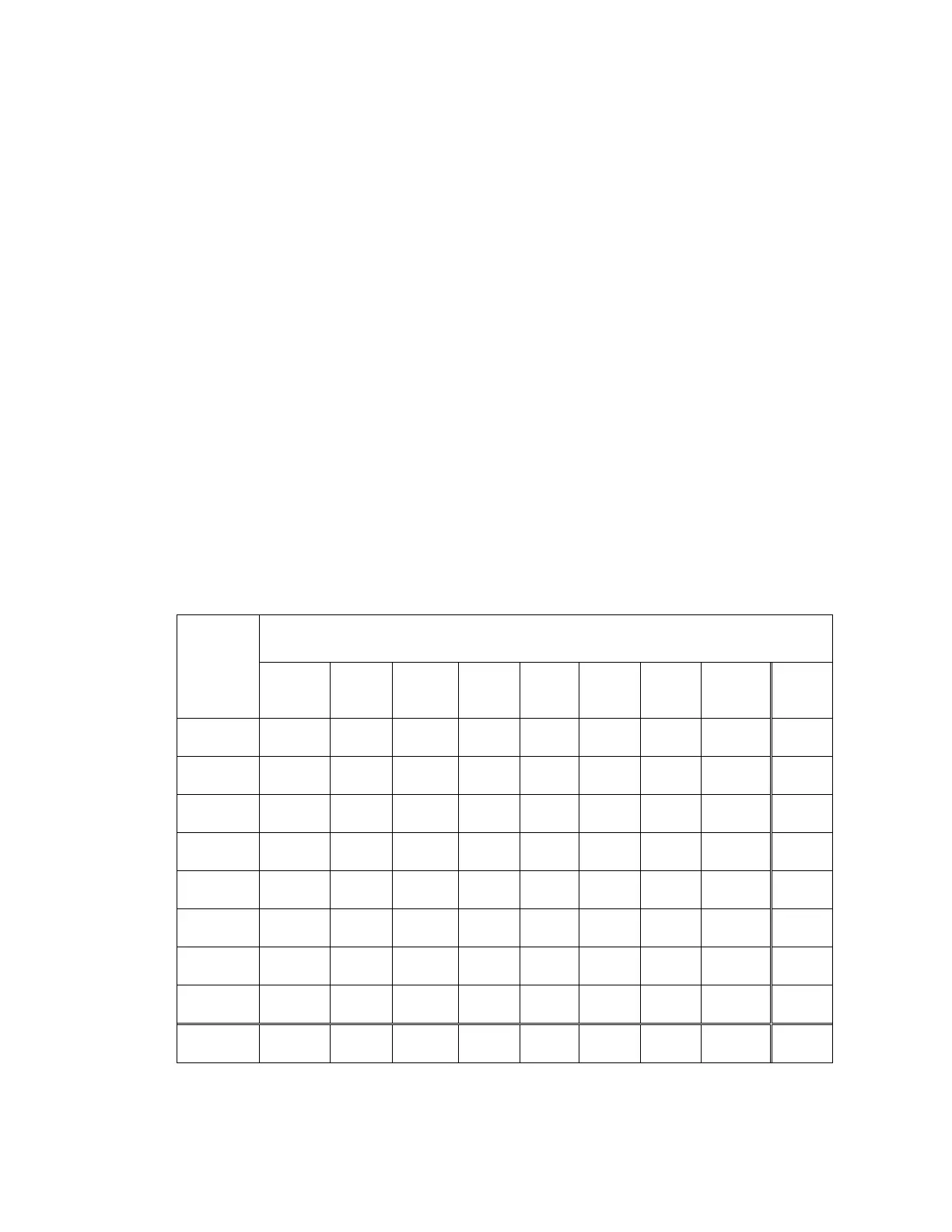
Table 18: Laboratory ThinPrep 5000 Diagnosis vs. Laboratory ThinPrep 2000 Diagnosis for
First Pair of Cytotechnologist/Pathologist (Combined Sites)
Lab
ThinPrep
5000
Diagnosis
Lab ThinPrep 2000 Diagnosis
UNSAT NILM ASC-
US
AGUS LSIL ASC-H HSIL Cancer Total
UNSAT
31 9 1 1 42
NILM
9 624 32 2 4 3 2 676
ASC-US
3 23 59 3 33 10 1 132
AGUS
1 5 7 1 3 3 20
LSIL
6 19 1 111 9 14 160
ASC-H
6 7 2 9 27 12 63
HSIL
2 12 16 109 2 141
Cancer
3 23 26
Total
44 673 119 16 170 66 144 28 1260
 Loading...
Loading...