ThinPrep™ 5000 System Instructions for Use English AW-22289-001 Rev. 003 11-2021 21/36
Reference Diagnosis by Adjudication Review
After all slides in the study were reviewed, all ThinPrep 2000 and ThinPrep 5000 slides were
subject to an adjudication review. Adjudication was done at a facility that was not one of the
study sites conducting the study. Slides for adjudication were evenly divided between three (3)
adjudication panels each consisting of one (1) cytotechnologist and three (3) independent
pathologists. Each adjudication panel was blinded to the original review diagnosis for all slides
and each independent pathologist within each panel was also blinded to other adjudicator’s
diagnoses for all slides. Adjudication consensus agreement was obtained for each slide
reviewed. Consensus agreement was achieved when at least two (2) of the three (3) pathologists
from a panel rendered an identical diagnosis. In cases where consensus agreement was not
achieved the panel members were brought together at a multi-head microscope to review the
slides together and come to a consensus diagnosis. For each specimen, an adjudicated
diagnosis for the ThinPrep 2000 slide and an adjudicated diagnosis for the ThinPrep 5000 slide
were obtained.
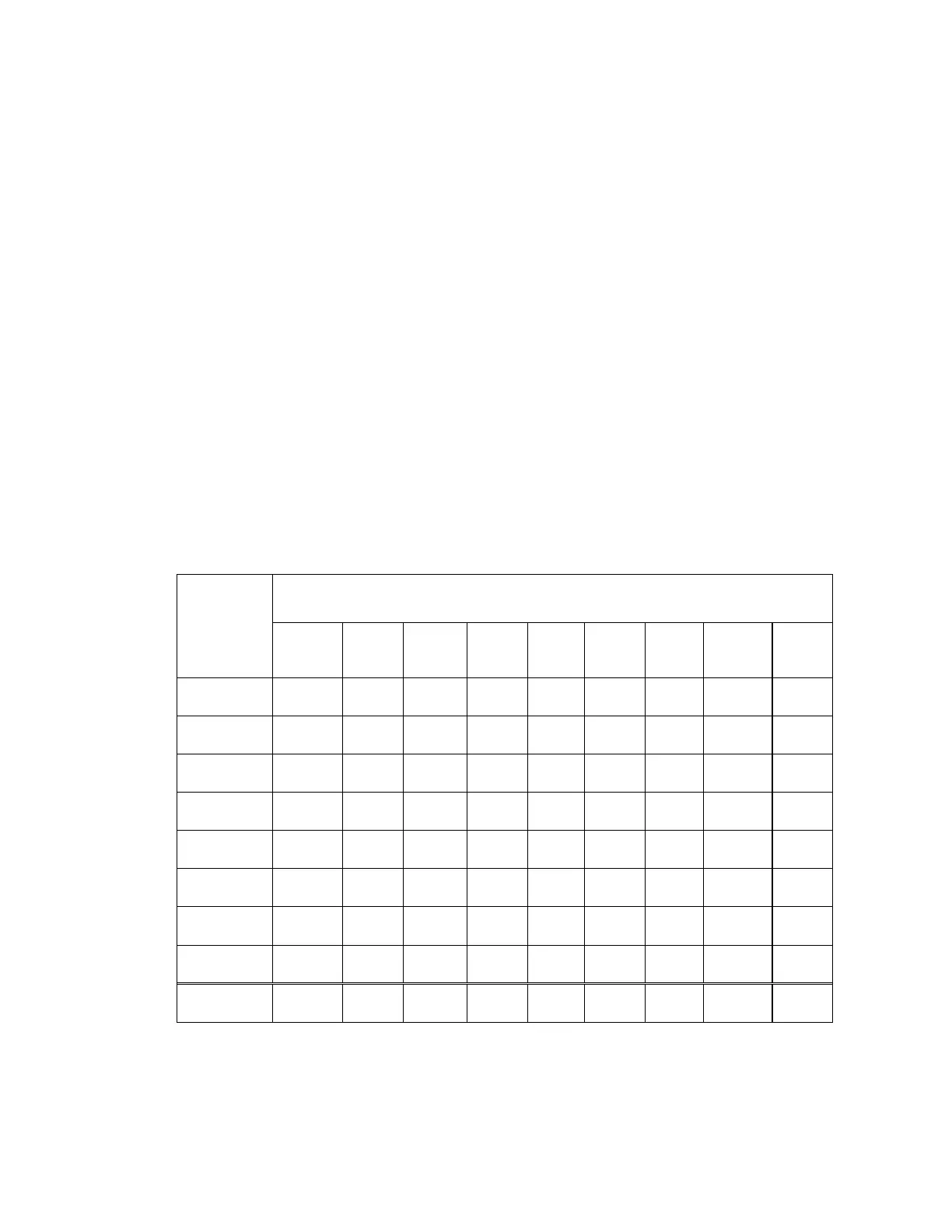
Table 19: Adjudicated ThinPrep 5000 Diagnosis vs. Adjudicated ThinPrep 2000 Diagnosis
(Combined Sites)
Adjudicated
ThinPrep
5000
Diagnosis
Adjudicated ThinPrep 2000 Diagnosis
UNSAT NILM ASC-
US
AGUS LSIL ASC-H HSIL Cancer Total
UNSAT
14 8 1 23
NILM
12 696 39 8 9 2 4 770
ASC-US
33 48 4 26 7 4 122
AGUS
4 1 6 4 3 18
LSIL
12 20 135 3 10 180
ASC-H
7 4 2 6 7 11 37
HSIL
7 1 9 8 66 1 92
Cancer
2 16 18
Total
26 760 119 21 185 28 101 20 1260
For each specimen, the Reference Diagnosis (RD) was considered as the most abnormal
diagnosis from the adjudicated diagnoses of the ThinPrep 2000 and ThinPrep 5000 slides. In the
 Loading...
Loading...