CLINITEK Status+ Analyzer Operator’s Guide 155
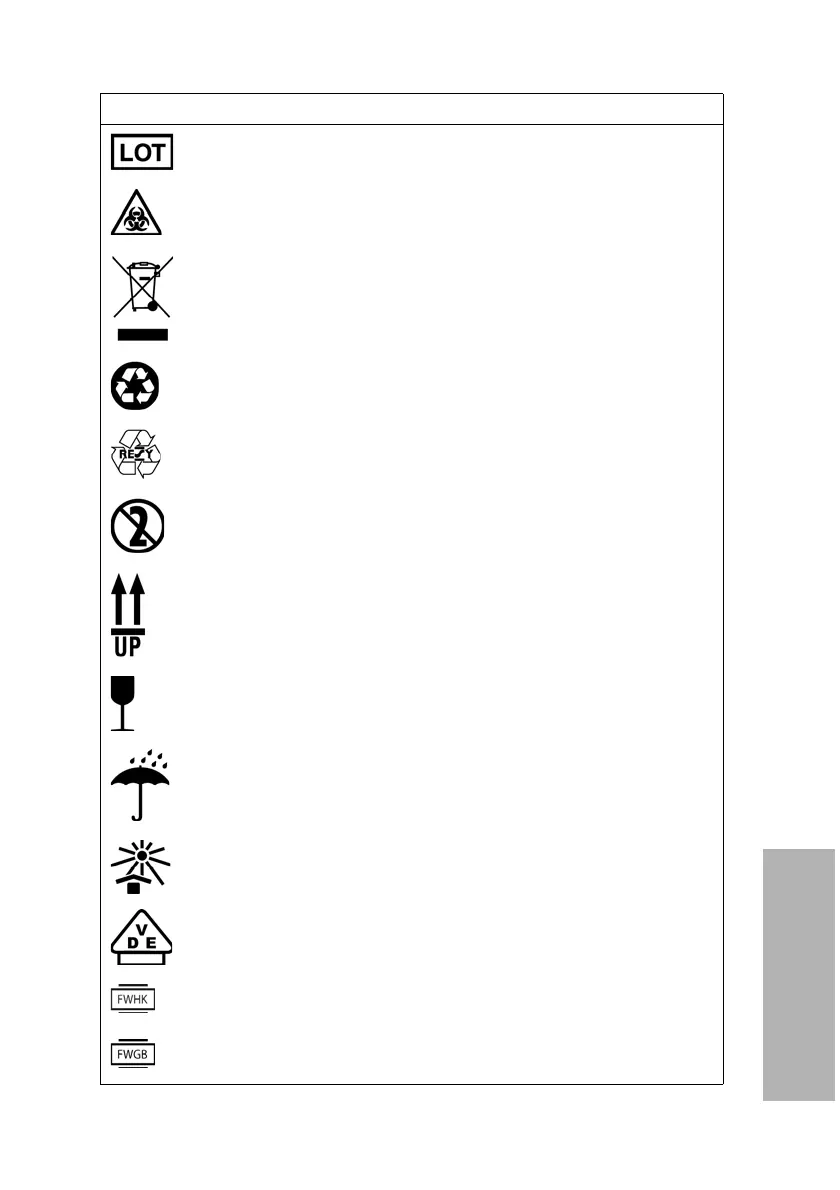
Symbols
Batch code
Biohazard
This equipment is classified as Waste Electrical and
Electronic Equipment under the European WEEE
Directive. It must be recycled or disposed of in accordance
with applicable local requirements
Printed on recycled materials
Indicates compliance with the RESY packaging standards
Do not reuse a reagent
Keep this way up
Fragile, handle with care
Keep dry
Keep away from sunlight and heat
VDE Testing and Certification Institute – Germany
Manufacturer’s mark (FWHK) and manufacturing location
(Hong Kong)
Manufacturer’s mark (FWGB) and manufacturing location
(Geratebau, Germany)
Symbol Description
 Loading...
Loading...











