BD BACTEC™ FX40 Instrument User’s Manual
14
Database
The database maintains the test measurements for each vial, vial identification and association
data, and patient demographic information. Data is stored in the BD BACTEC™ FX40 database in
both standalone and BD EpiCenter™ configurations. In addition, associated instrument errors, and
operating conditions (e.g., test times, instrument temperature, etc.) are stored in the database.
The database stores vial results for up to 60 days after removal from the instrument (readings are
maintained for up to 14 days).
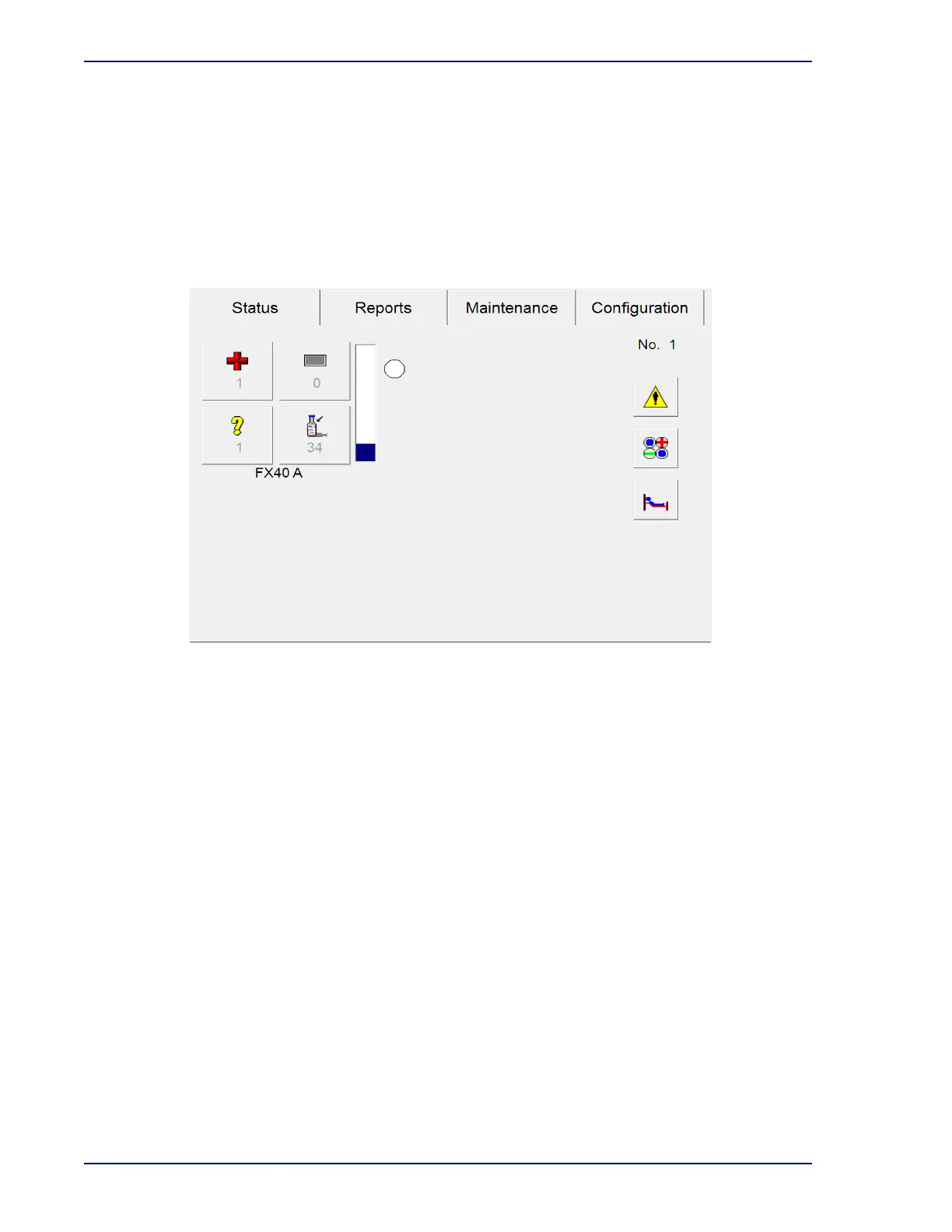
Figure 1-3 – BD BACTEC™ FX40 Status Display
1.3.13 Media Overview
Several media are available for use with the BD BACTEC™ FX40 system. These include:
BD BACTEC™ Standard/10 Aerobic /F
Indicated for 3.0 to 10.0 mL (8.0 to 10.0 mL optimal) blood volume.
BD BACTEC™ Plus Aerobic /F
Contains resins for antibiotic neutralization. Indicated for 3.0 to 10.0 mL (8.0 to 10.0 mL optimal)
blood volume.
BD BACTEC™ Standard Anaerobic /F
Indicated for 3.0 to 7.0 mL (5.0 to 7.0 mL optimal) blood volume.
BD BACTEC™ Peds Plus™ /F
Optimized for use with pediatric patients and for low blood volume specimens 1.0 to 3.0 mL (range
of 0.5 to 5.0 mL). Contains resins for antibiotic neutralization.
BD BACTEC™ Plus Anaerobic /F
Contains resins for antibiotic neutralization. Indicated for 3.0 to 10.0 mL (8.0 to 10.0 mL optimal)
blood volume.
 Loading...
Loading...