GE H
EALTHCARE
D
IRECTION
GA091568, R
EVISION
5 VIVID E9 S
ERVICE
M
ANUAL
1 - 28 Section 1-5 - Labels locations
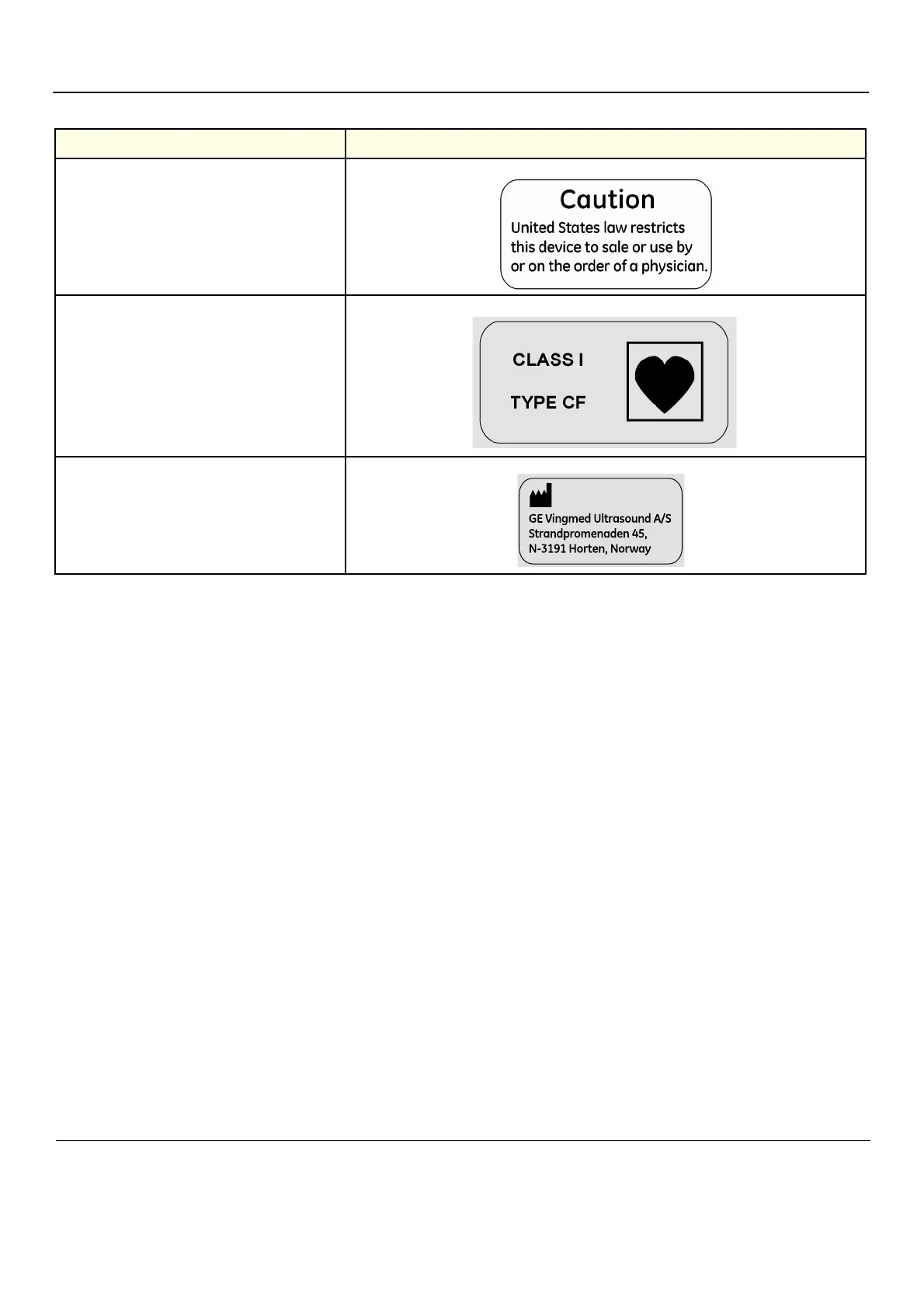
CAUTION
United States law restricts this device to sale or use
by or on the order of a physician.
CLASS I
The VIVID E9 ultrasound unit is a Class I device,
type CF, according to Sub-clause 14 of IEC60601-1
(1988).
TYPE CF
Equipment Type CF (heart in the box symbol) IEC
878-02-05 indicates equipment having a floating
applied part having a degree of protection suitable
for direct cardiac contact.
MANUFACTURER
Table 1-17 Label on Rear Cover - detailed descriptions (cont’d) sheet 3 of 3
DESCRIPTION ILLUSTRATION
 Loading...
Loading...