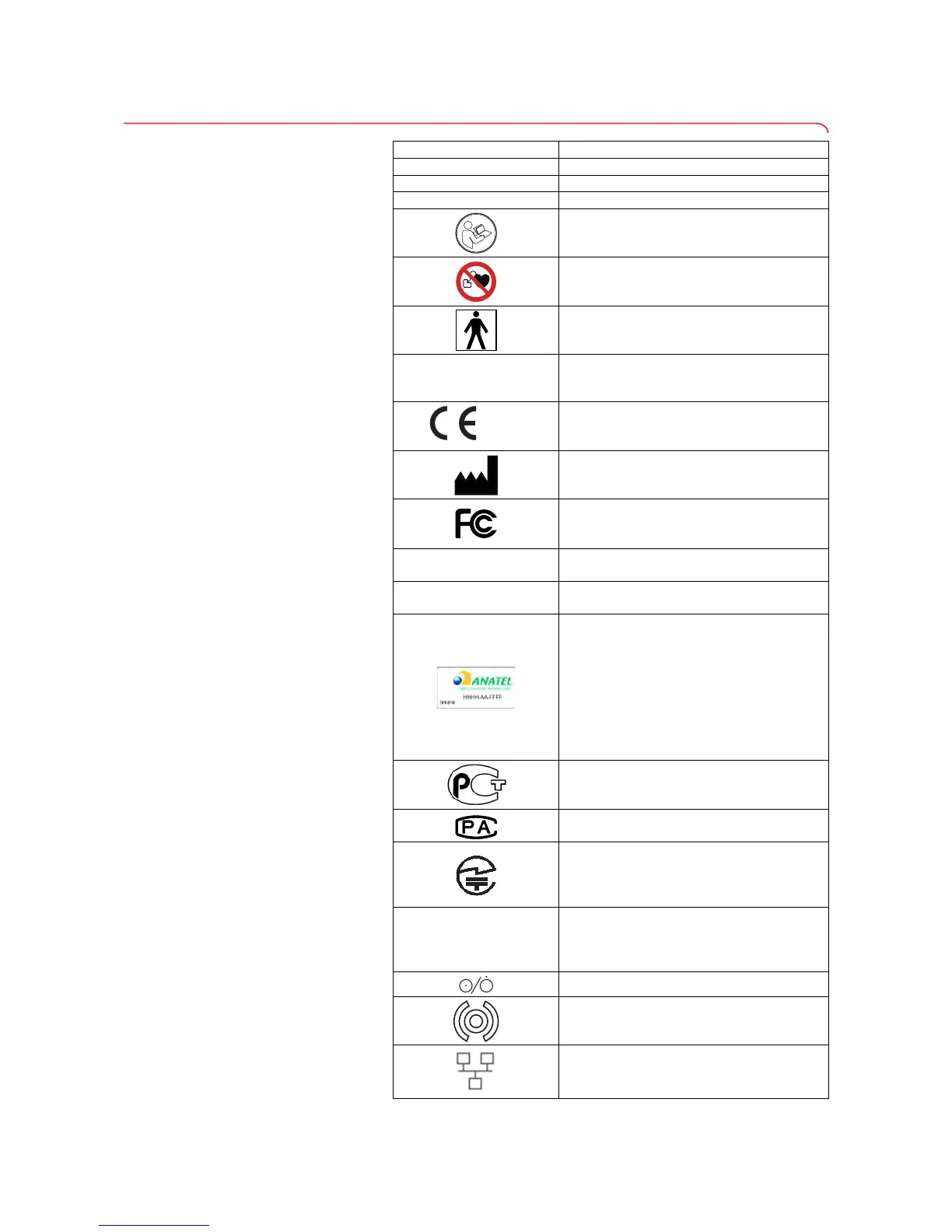
3.6 Identification on the device and on the type plate
Text/symbol Meaning
Fabr Manufacturer
Mod Model number
S/N Serial number, consecutive
Follow Instructions for Use
Do not use device on individuals with
cardiac pacemakers or implanted
defibrillators
Medical electrical device, type BF
Lithium-ion rechargeable battery
Device complies with EC directives
• 0123: appointed office for medical devices:
TÜV SÜD Product Service
Name and address of manufacturer, date of
manufacture
Symbol of the US Federal Communications
Commission (FCC)
FCC ID
Device license number from the Federal
Communications Commission (FCC)
IC
Device license number from Industry
Canada
The device meets the requirements of the
Agência Nacional de Telecomunicações
[National Telecommunications Agency
(ANATEL), Brazil]. Details of the wireless
device license:
- HHHH: license number of device
- AA: year of license
- FFFF: identification number of the
manufacturer
The device meets the regulatory
requirements of GOST R certification
(Russia)
License number of the
Chinese Pharmaceutical Association (CPA)
The device meets the regulatory
requirements on wireless equipment in
Japan. License number:
VORL.202WW09118012
xxx-yyy V ~
xx-yy Hz
xx A
Type plate for power supply socket:
• Permitted supply voltage
• Permitted power supply frequency
•Power consumption
ON/OFF key
Inductive charging interface
LAN interface
 Loading...
Loading...