Table A-3. Guidance and Manufacturer’s Declaration – Electromagnetic Immunity For Equipment and
Systems That Are Not Life-Supporting (per IEC 60601-2 Clause 6.8.3.201 b Table 204)
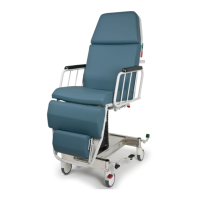
The STERIS 4095 General Surgical Table and related components are intended for use in the electromagnetic environment
specified below. The Customer or the user should ensure this STERIS 4095 General Surgical Table is used in such an
environment.
Immunity test IEC 60601
Test Level
Compliance
Level
Electromagnetic Environment–
Guidance
Conducted RF
IEC 61000-4-6
Radiated RF
IEC 61000-4-3
3 Vrms (Outside ISM)
6Vrms (In ISM Bands)
150 kHz to 80 MHz
3 V/m
80 MHz to 2.7 GHz
(V1) = 3 Vrms
(V2) = 6 Vrms
(V3) = 10 Vrms
(E1) = 3 V/m
Portable and mobile RF communications equipment
should be separated from the STERIS 4095 General
Surgical Table, including cables, no less than the
distance calculated/listed below:
D=(3.5/V1)(Sqrt P)
D=(12/V2)(Sqrt P)
D=(12/E1)(Sqrt P)
80 to 800 MHz
D=(23/E1)(Sqrt P)
800 MHz to 2.7 GHz
where P is the max power in watts and D is the
recommended separation distance in meters.
Field strengths from fixed transmitters, as
determined by an electromagnetic site survey,
should be less than the compliance levels (V1 and
E1).
Interference may occur in the vicinity of equipment
containing a transmitter.

 Loading...
Loading...