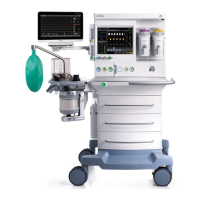
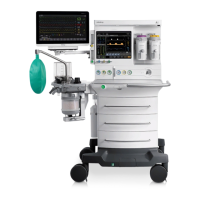
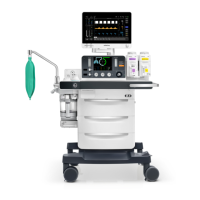
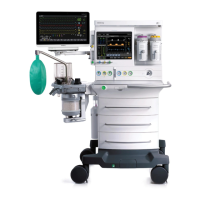
1-4
equipment in any single area.
Due to the size and weight of the equipment, it should only be moved by qualified
personnel.
Overloading machine may cause tipping. Equipment attached to the side of the
equipment should be within the rated weights to prevent dumping of the machine.
Excess load may cause a tip hazard while moving the equipment. Before moving,
remove all equipment from the top shelf and all monitoring equipment installed to
the side of the equipment. Use care when moving the equipment up or down a
slope, around a corner, and across threshold. Do not attempt to roll the equipment
over hoses, cords, or other obstacles.
Leaks or internal venting of sampled gas may affect accuracy. Perform proper
preoperative tests to ensure that the equipment is operating properly. Leaky
circuits can not be used.
Connecting the equipment's exhaust port to the hospital’s waste gas scavenging
system is strongly recommended to prevent exposure of hospital personnel to the
waste gas.
Pins of connectors identified with the ESD warning symbol should not be touched.
Connections should not be made to these connectors unless ESD precautionary
procedures are used.
Operation of the equipment below the minimum flow values may cause inaccurate
results.
This equipment/system is intended for use by healthcare professionals only. This
equipment/system may cause radio interference or may disrupt the operation of
nearby equipment. It may be necessary to take mitigation measures, such as
reorienting or relocating the equipment, or shielding the location it was placed.
Ensure that an independent means of ventilation (e.g. a self-inflating manual
resuscitator with mask) is available whenever the equipment is in use.
The use of accessories with damaged packaging may cause biocontamination or
failure. The operator should check the integrity of accessory packaging before use.
Before using the anesthesia system after cleaning or disinfecting, power on the
system and follow the on-screen prompts to perform leak test and compliance test.
Improperly cleaned materials may result in biocontamination. Use a cleaning and
disinfection schedule that conforms to your institution's disinfection and
risk-management policies.
Refer to the material safety data sheet as applicable.
Refer to the operation and maintenance manuals of all disinfection equipment.
User should follow the recommended disinfection routine for this equipment and
any reusable accessories.
Oxygen, when present in high concentrations, can significantly increase the chance
of fire or explosion. Oil and grease may be ignited at the same time. Therefore, oil
and grease should not be used where oxygen enrichment may occur.
Use of lubricants not recommended by Mindray may increase the danger of fire or
explosion. Please use lubricants as approved by Mindray.
Low-pressure regulators and flow-meters are susceptible to high pressure, and
may burst if improperly maintained or disassembled while under pressure.
Changing or disassembling connectors should be performed only by qualified
 Loading...
Loading...