8-19
Figure B
8.21 Disassemble the Motherboard
8.21.1 Prepare for Disassembly
8.21.1.1 Tools
During parts disassembly and replacement, the following tools may be required:
Phillips screwdriver
8.21.1.2 Preparations
Before disassembly,
Make sure that the anesthesia machine is turned off and disconnected from the A/C power
source.
Maneuver the anesthesia machine to an appropriate location and then apply the brake.
8.21.2 Pre-disassembly
1. Refer to 8.1.1Open the Service Door to open the service door.
2. Refer to 8.6Disassemble the Monitoring Board to remove the monitoring board assembly.
3. Refer to 8.8Disassemble the DC-DC Board to remove the DC-DC board assembly.
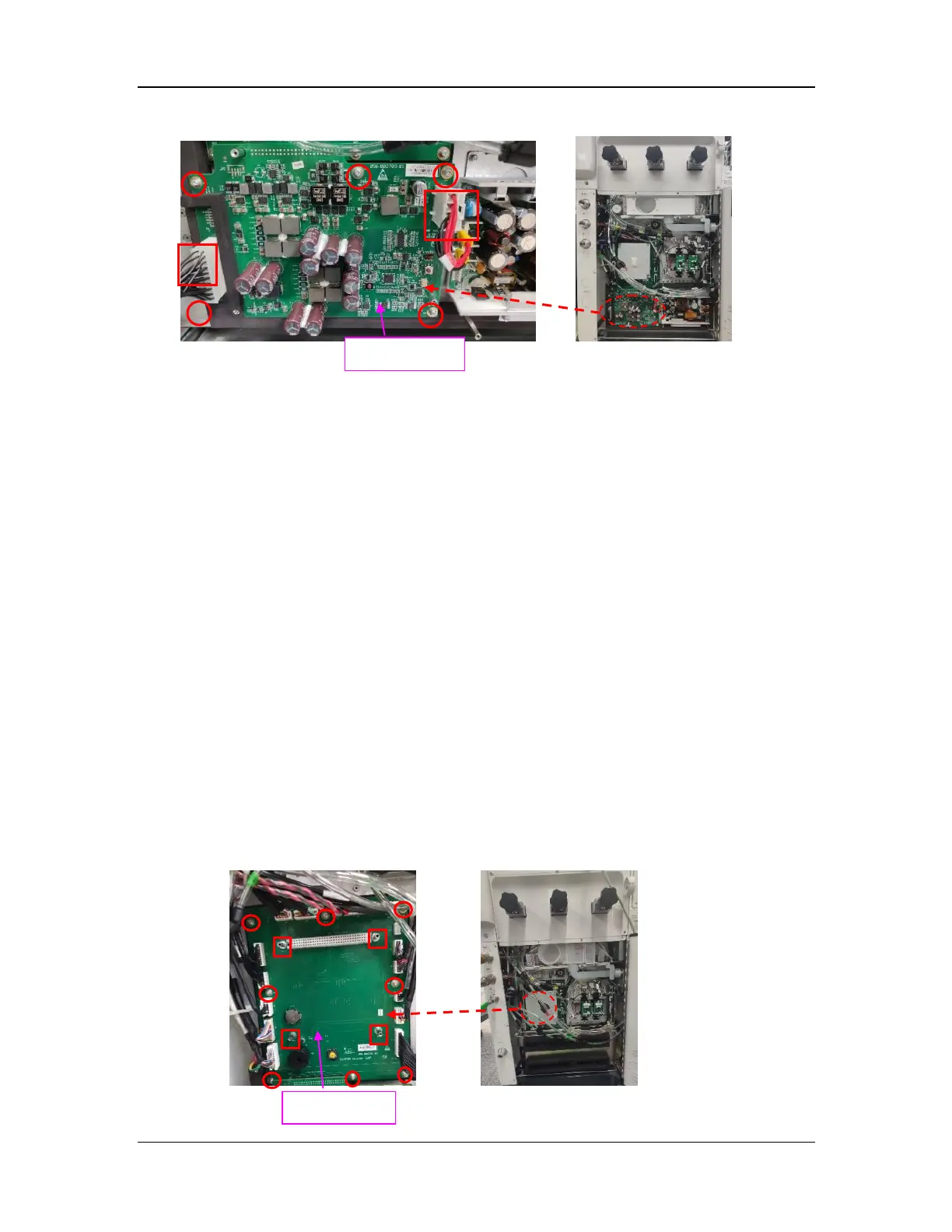
1.9.2 Remove the Motherboard FRU
Disconnect the connection lines from the motherboard. Then remove the eight screws (marked by
○ in the figure) and four stud screws (marked by □ in the figure) from the motherboard FRU with
the Phillips screwdriver to remove the motherboard.

 Loading...
Loading...