Solvent miscibility C-5
How to use miscibility numbers
Use miscibility numbers (M-numbers) to predict the miscibility of a liquid
with a standard solvent (see the table titled “Solvent miscibility” on page C-3).
To predict the miscibility of two liquids, subtract the smaller M-number value
from the larger M-number value.
• If the difference between the two M-numbers is 15 or less, the two
liquids are miscible in all proportions at 15 °C.
• A difference of 16 indicates a critical solution temperature from 25 to
75 °C, with 50 °C as the optimal temperature.
• If the difference is 17 or greater, the liquids are immiscible, or their
critical solution temperature is above 75 °C.
Some solvents prove immiscible with solvents at either end of the lipophilicity
scale. These solvents receive a dual M-number:
• The first number, always lower than 16, indicates the degree of
miscibility with highly lipophilic solvents.
• The second number applies to the opposite end of the scale. A large
difference between these two numbers indicates a limited range of
miscibility.
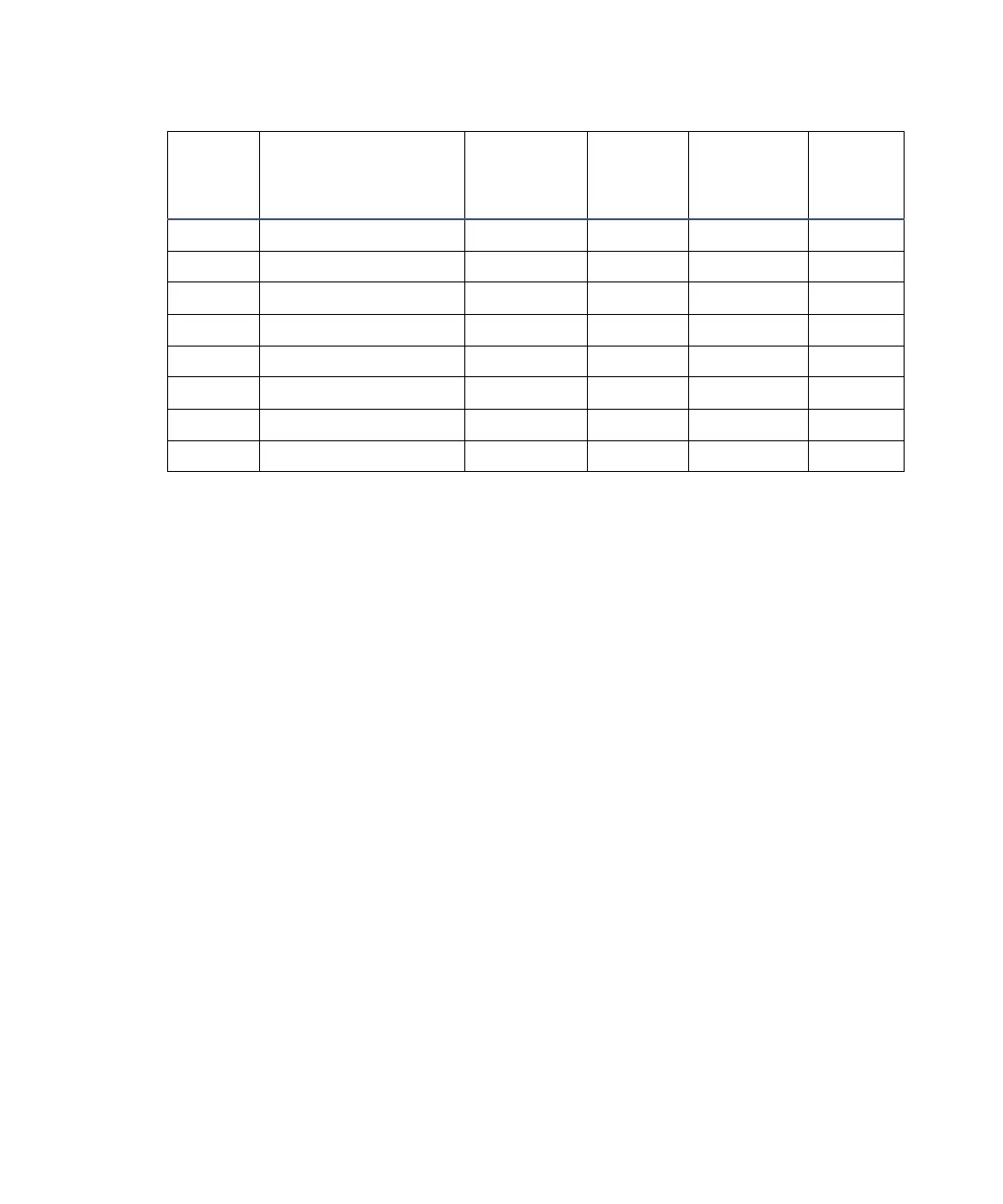
5.7 Methoxyethanol 1.72 124.6 13 ––
6.2 Acetonitrile 0.37 81.6 11, 17 210
6.2 Acetic acid 1.26 117.9 14 ––
6.4 Dimethylformamide 0.90 153.0 12 ––
6.5 Dimethylsulfoxide 2.24 189.0 9 ––
6.6 Methanol 0.60 64.7 12 210
7.3 Formamide 3.76 210.5 3 ––
9.0 Water 1.00 100.0 –– ––
Solvent miscibility (Continued)
Polarity
index
Solvent
Viscosity
CP, 20 °C
Boiling
point °C
(1 atm)
Miscibility
number
(M)
λ Cutoff
(nm)
 Loading...
Loading...