10 Calculation Methods 10-3
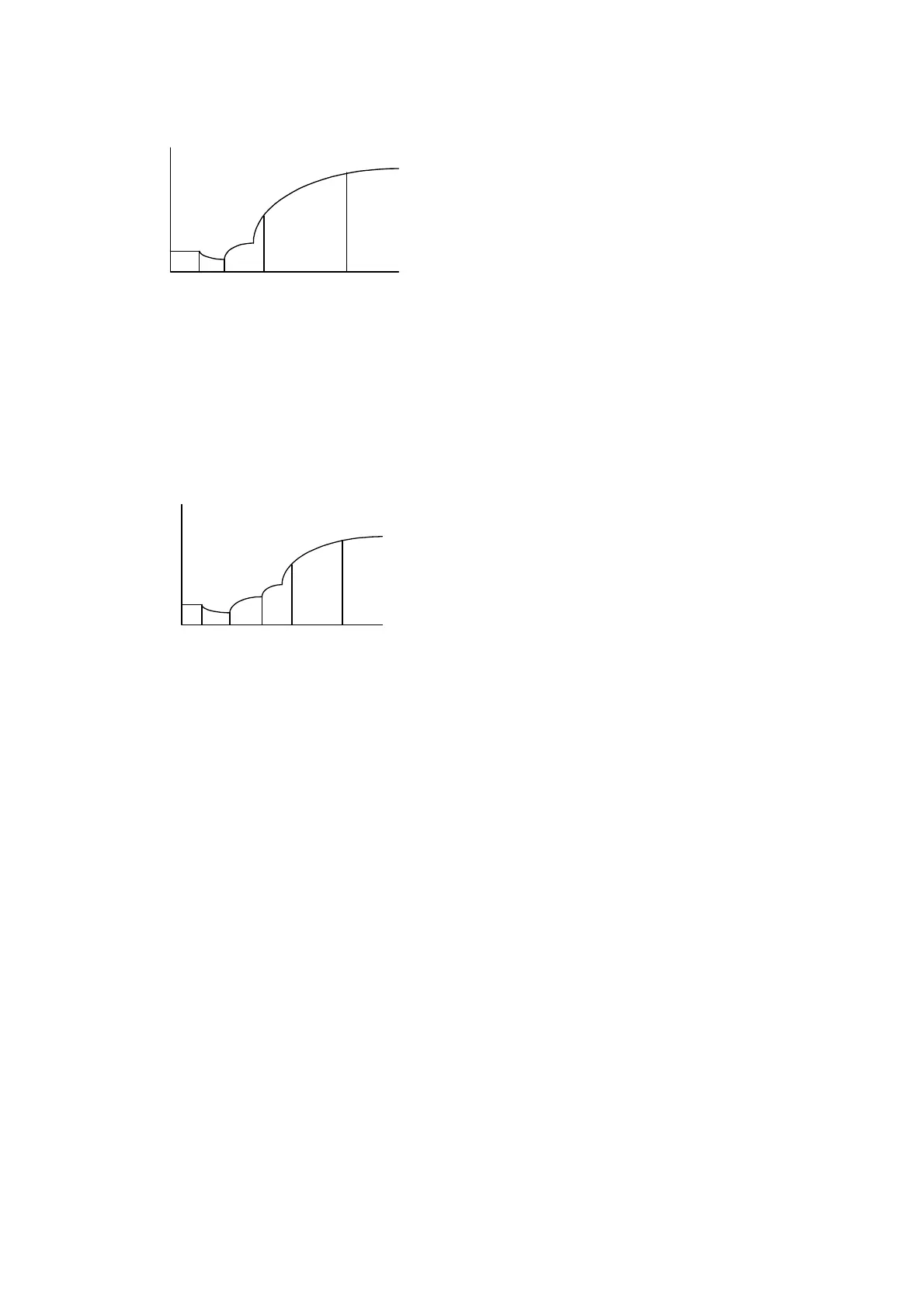
Figure 10-3 Single-reagent Fixed-time Reaction Curve
A
tt1 t2
t3 t4
As shown in Figure 10-3,
1
t is the time when the reagent is added and
2
t is the time
when the sample is added. The reaction starts when they are mixed. From
3
t
the
reaction becomes steady and
4
t is the time to stop monitoring the reaction.
2
t to
3
t
is
the lag period, and the absorbance readings are respectively taken at
3
t
and
4
t .
Figure 10-4 Double-reagent Fixed-time Reaction Curve
A
tt1 t2 t3 t4
t5
As shown inFigure 10-4
1
t is the time when the first reagent is added, and
2
t is the time
when the sample is added, and then the mixture absorbance reading is taken after they
are mixed.
3
t
is the time when the second reagent is added, then the reaction starts
when they are mixed. At
4
t the reaction reaches equilibrium, and
5
t
is the time to stop
monitoring the reaction.
2
t to
3
t
is the incubation period, and
3
t
to
4
t is the delay
period. The absorbance readings are respectively taken at
4
t and
5
t
.
The fixed-time reaction is demanding more technically than the equilibrium method.
Because reaction rate is measured at two different points, all the factors that affect
reaction rate, such pH, temperature, and amount of enzyme, must be kept constant from
one assay to the next, as must the timing of the two measurements. A reference solution
of the substrate must be used for calibration.
10.1.3 Kinetic
For the kinetic method (namely, zero-order kinetic or continuous-monitoring method), the
reaction velocity is not related to the substrate concentration and remains constant in the
reaction process. As a result, for a given wavelength, the absorbance of the analytes
changes evenly, and the change rate (
∆
A/min) is directly proportional to the activity or
concentration of the substrate. The kinetic method is usually used to measure enzyme
activity.
In fact, it is impossible for the substrate concentration to be high enough, and the reaction
will be no longer a zero-order reaction when the substrate is consumed to a certain
degree. Therefore, the theory only stands within certain period. In addition, the reaction
can become steady only after a certain period of time, because the reaction is
 Loading...
Loading...