15
Spectroquant
®
photometers
Release 06/2014
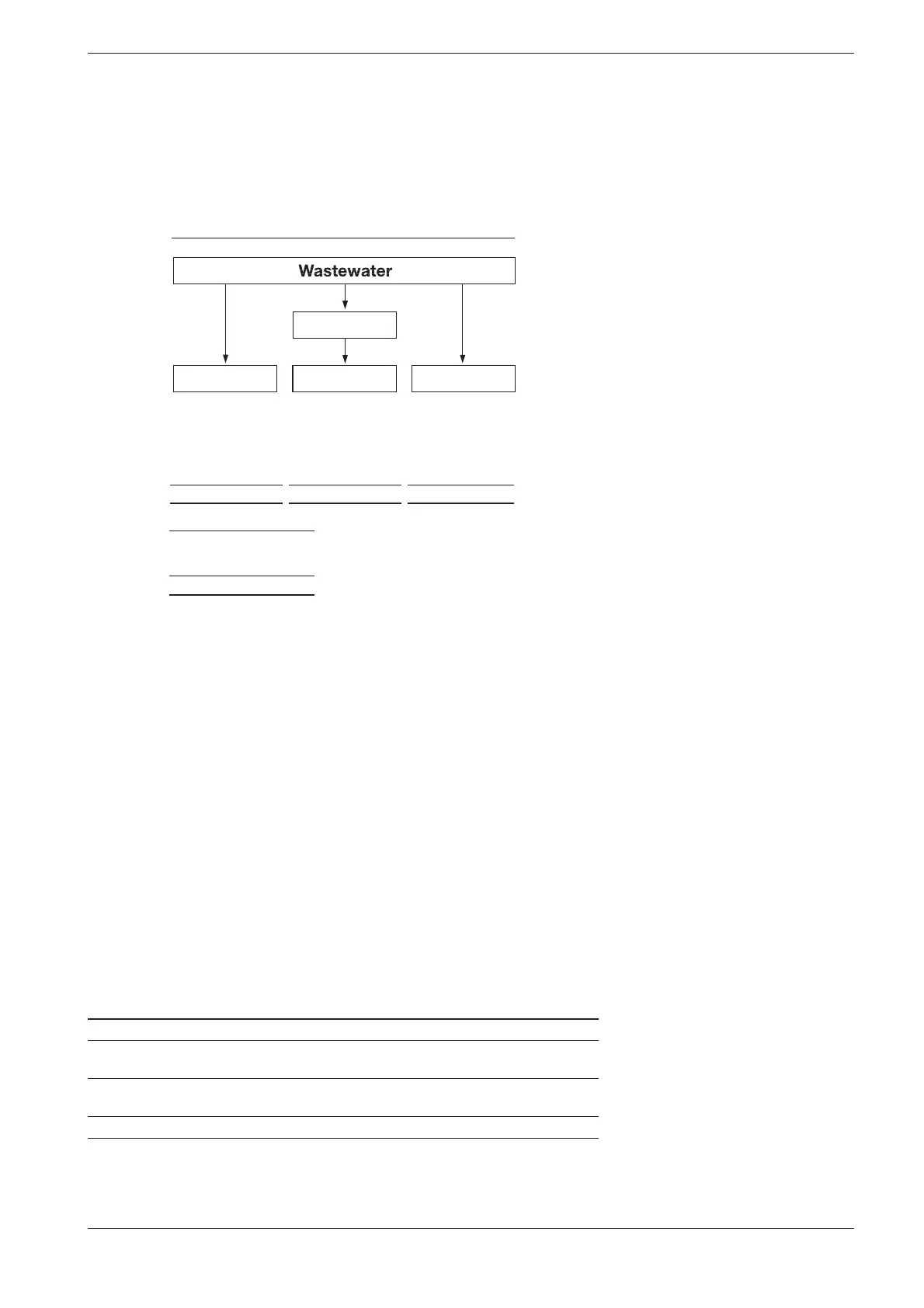
The manner in which the sample is pretreated en ables the three pro-
portions to be dis tinguished from each other. This can be illustrated using
a copper-containing waste water sample as an example.
Decomposition converts the substance to be deter mined into an ana-
lyzable form. In most cases, de composition agents take the form of acids
in com bination with oxidiz ing agents; in exceptional cases (e. g. in the
determination of total nitrogen) an alka line decomposition is more effec-
tive. The type of decomposition procedure used de pends on the analyte to
be determined and the sample matrix.
3 Sample Preparation
Example
Filtration
Filtration
Decomposition
Decomposition
The ready-to-use sample-decomposition products
Spectro
quant
®
Crack
Set 10 and 20 are suited for the preparation of the sample materials for
the determinations stated in the table.
The decomposition processes are carried out in the
Spectro
quant
®
ther-
moreactor (capacity: 12 or 24 decomposition cells) at 120 °C or, respec-
tively, 100 °C. Details regarding the heating times and further treatment
can be found in the package inserts contained in the Spectroquant
®
Crack Set packs.
Determination of Sample preparation with
Total phosphorus* Crack Set 10 / 10C**
Total chromium* Crack Set 10 / 10C
[= sum of chromate and chromium(III)]
Total metal Crack Set 10 / 10C
[= sum of free and complex-bound metal]
Total nitrogen* Crack Set 20
* The decomposition reagents are already contained in the packs of the respective cell tests.
** Decomposition cells are included in the pack; empty cells are required for the decomposition for
Crack Sets 10 and 20.
Total content Dissolved proportion Dissolved proportion
Solid Substances
Cu(OH)
2
Complexes Cu-EDTA Complexes Cu-EDTA
Ions Cu
2+
Ions Cu
2+
Ions Cu
2+
Result A Result B Result C
Proportion:
Ionogenic = C
Complex = B– C
Solid Substances = A– B
Total content = A
 Loading...
Loading...