22
Spectroquant
®
photometers
Release 06/2014
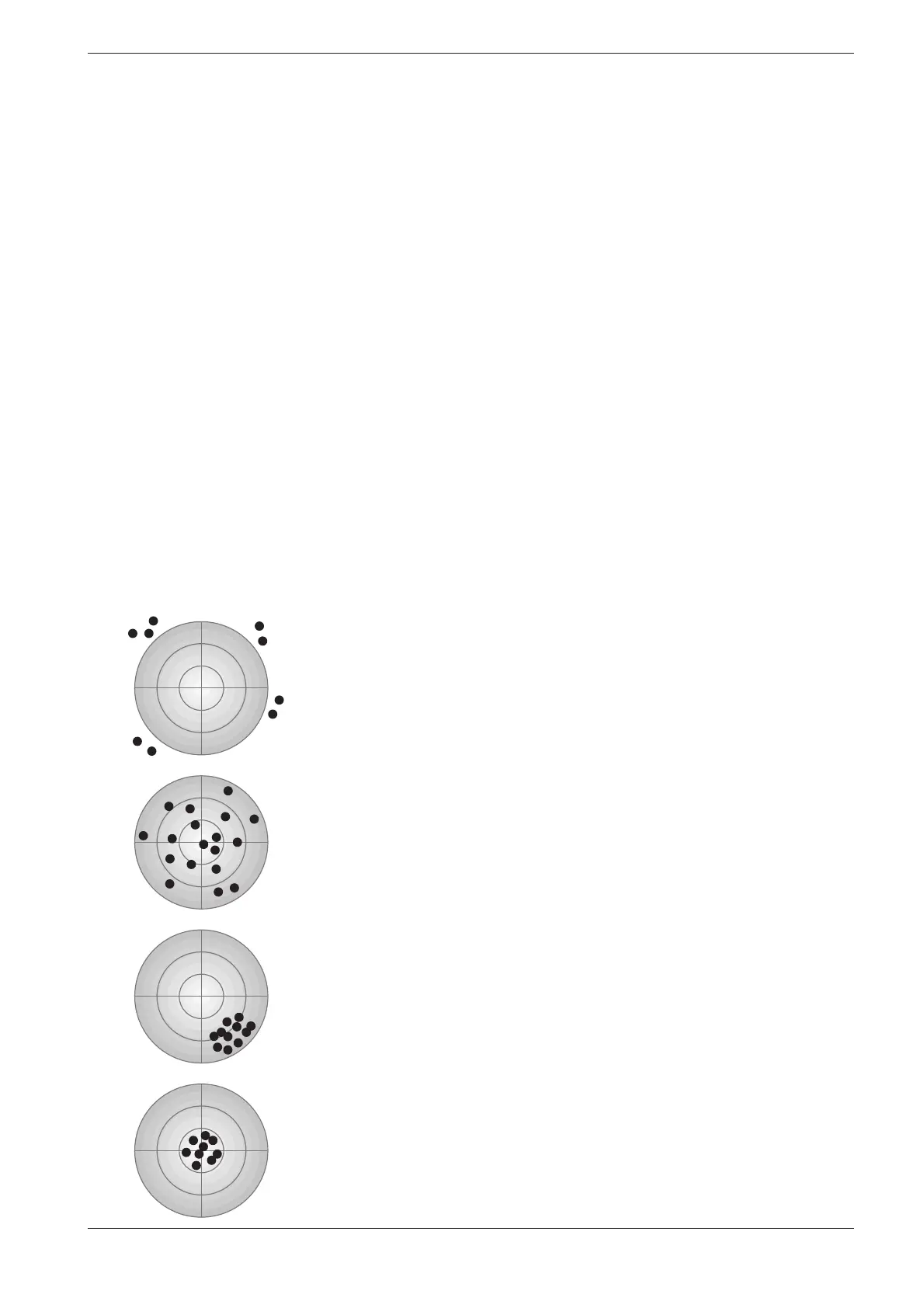
Accuracy: poor
Precision: poor
Major errors have been made!
Accuracy: good
Precision: poor
Calculation of the mean values from at least three – or better even more –
parallel determina tions yields an approximation of the true value.
Accuracy: poor
Precision: good
The high degree of precision mis takenly indicates a correct value!
Accuracy: good
Precision: good
The ideal objective!
5 Analytical Quality Assurance (AQA)
The following diagram illustrates the aspects of accuracy and precision:
5.4 Definition of Errors
It is obvious that measurement results as a rule may be associated with
errors. This applies equally to standardized methods of analysis (reference
methods) and to rou tine analysis. The discovery and the minimization of
errors must be the objective here.
A distinction is made between systematic errors and random errors.
Systematic errors are present when all the results of an analysis deviate
from the true value with the same algebraic sign. Examples here include:
a wrong sample volume, a wrong pH, a wrong reaction time, a sample-
matrix influence, etc. Systematic errors thus affect the accuracy of the
method of analysis.
Accuracy = Deviation of the measured concentration from the true con-
centration
Random errors manifest themselves in the form of a wide range of devia-
tion of the results of a given sample. These can be kept to a minimum by
ensuring good operat ing techniques and multiple determina tion with cal-
culation of the mean values. Ran dom errors make the result of the analy-
sis unreliable; they influence the precision.
Precision = Dispersion of the results among each other
 Loading...
Loading...