Patient monitor User’s manual
1
Appendix A Product Specifications
A.1 Safety Specifications
According to the MDD 93/42/EEC, the monitor is Type Ⅱb equipment. Classified
according to the IEC60601-1 is as follows:
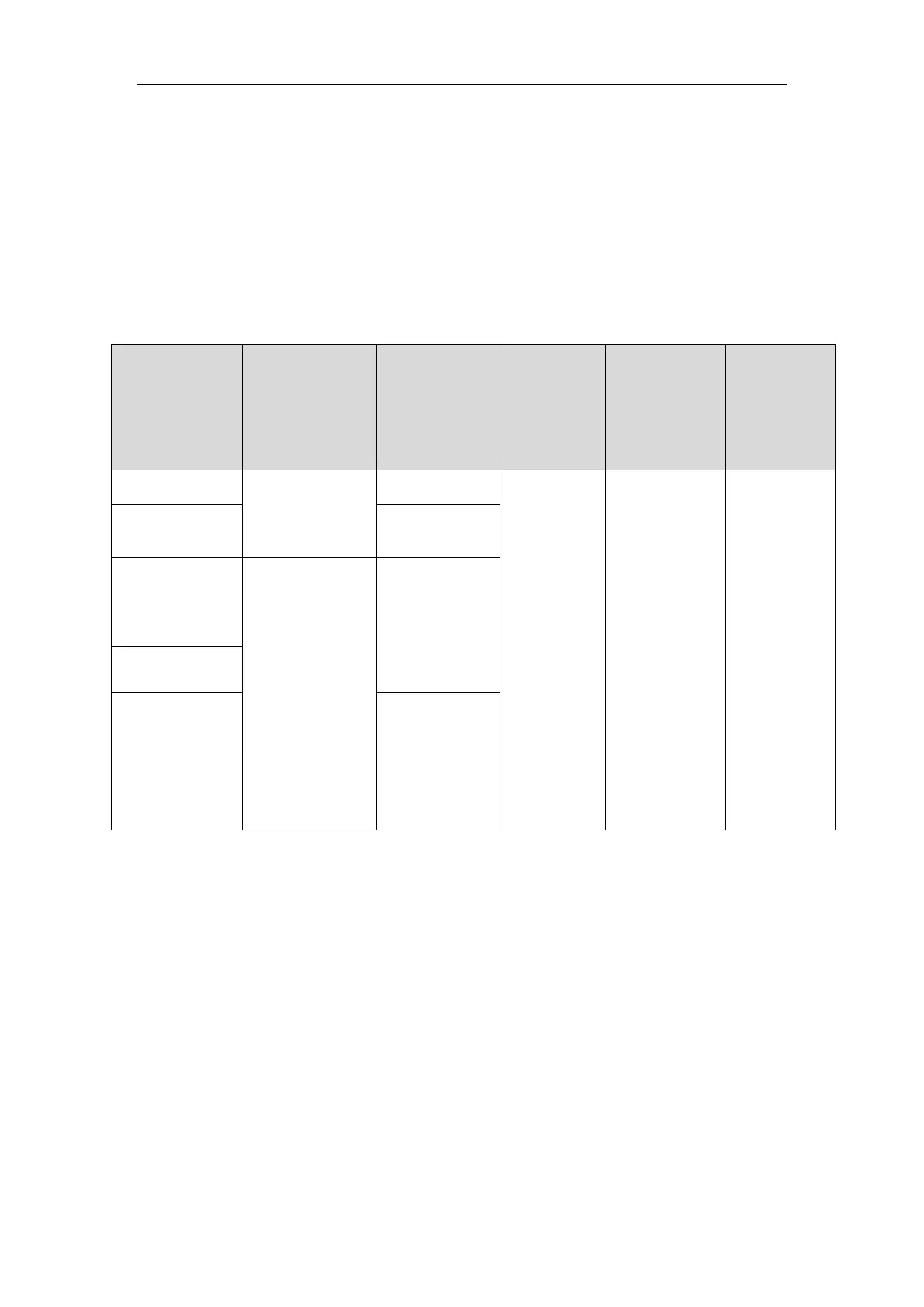
Parts
Classification
of protection
against
electric shock
Degree of
protection
against
electric
shock
Degree of
protection
against
ingress of
liquid
Degree of
protection
against
hazards of
explosion
Mode of
operation
Mainframe
I
No mark
IPX1
Not suitable
Continuous
Secondary
display
No mark
Temp Module
NA
Type CF
applied part
defibrillation
proof
IBP Module
SpO
2
Module
CO
2
Module
Type BF
applied part
defibrillation
proof
AG Module
Note:
I: Class I, internally and externally powered equipment.
When you doubt about the protecting earth integrality or protecting earth lead of
the equipment, you’d better change the equipment to internally powered equipment.
CF: Type CF applied part with defibrillation proof.
BF: Type BF applied part with defibrillation proof.
NA: Not applicable
Not suitable: Equipment is not suitable for use in the presence of flammable
anesthetic mixture with air or with oxygen or nitrous oxide.
 Loading...
Loading...