Roche Diagnostics
ii Operator’s Manual · Version 1.0
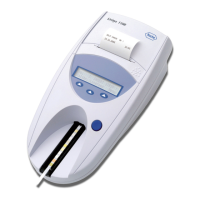
URISYS 2400
Revision History
Edition notice URISYS 2400 Operator’s Manual
This manual is for users of the URISYS 2400.
Every effort has been made to ensure that all the information contained in this
manual is correct at the time of printing. However, Roche Diagnostics GmbH
reserves the right to make any changes necessary without notice as part of
ongoing product development.
Any customer modification to the instrument will render the warranty or
service agreement null and void.
Intended use The URISYS 2400 is a fully automated urinalysis system intended for in vitro
qualitative or semi-quantitative determination of urine analytes, including
pH, leukocytes, nitrite, protein, glucose, ketones, urobilinogen, bilirubin, and
erythrocytes.
Copyrights © 2001, Roche Diagnostics GmbH. All rights reserved.
Trademarks The following trademarks are acknowledged:
, URISYS 2400 are registered trademarks of the Roche group. All other
trademarks that are mentioned in this manual are the property of the
respective trademark holders: KOVA® and Monovette®.
Instrument approvals The URISYS 2400 analyzer meets the requirements stated in
Directive 98⁄79⁄EC of the European Parliament and the Council of the
European Union (EU) on in vitro diagnostic medical devices. Furthermore,
the URISYS 2400 analyzer is manufactured and tested according to
International Standard IEC 1010-1, "Safety requirements for electrical
equipment for measurement, control, and laboratory use, Part 1: General
requirements". This International Standard is equivalent to the standards UL
3101-1 for the USA, CSA C 22.2 No. 1010.1 for Canada, and DIN EN 61010-1
for Germany.
The URISYS 2400 fulfils the EMC immunity requirements for laboratory use
equipment in industrial areas, according to the EMC standards EN 50082-2
and EN 61326-A1. According to EN 55011, the URISYS 2400 analyzer is a
device of limit class A.
Version Revision date
1.0 April 2001
Language Order No.
English
03184315.018

 Loading...
Loading...