108 User Information
Developing Methods
Developing Methods
This chapter discusses various parameters that can affect the
sensitivity, precision, and accuracy of an analysis.
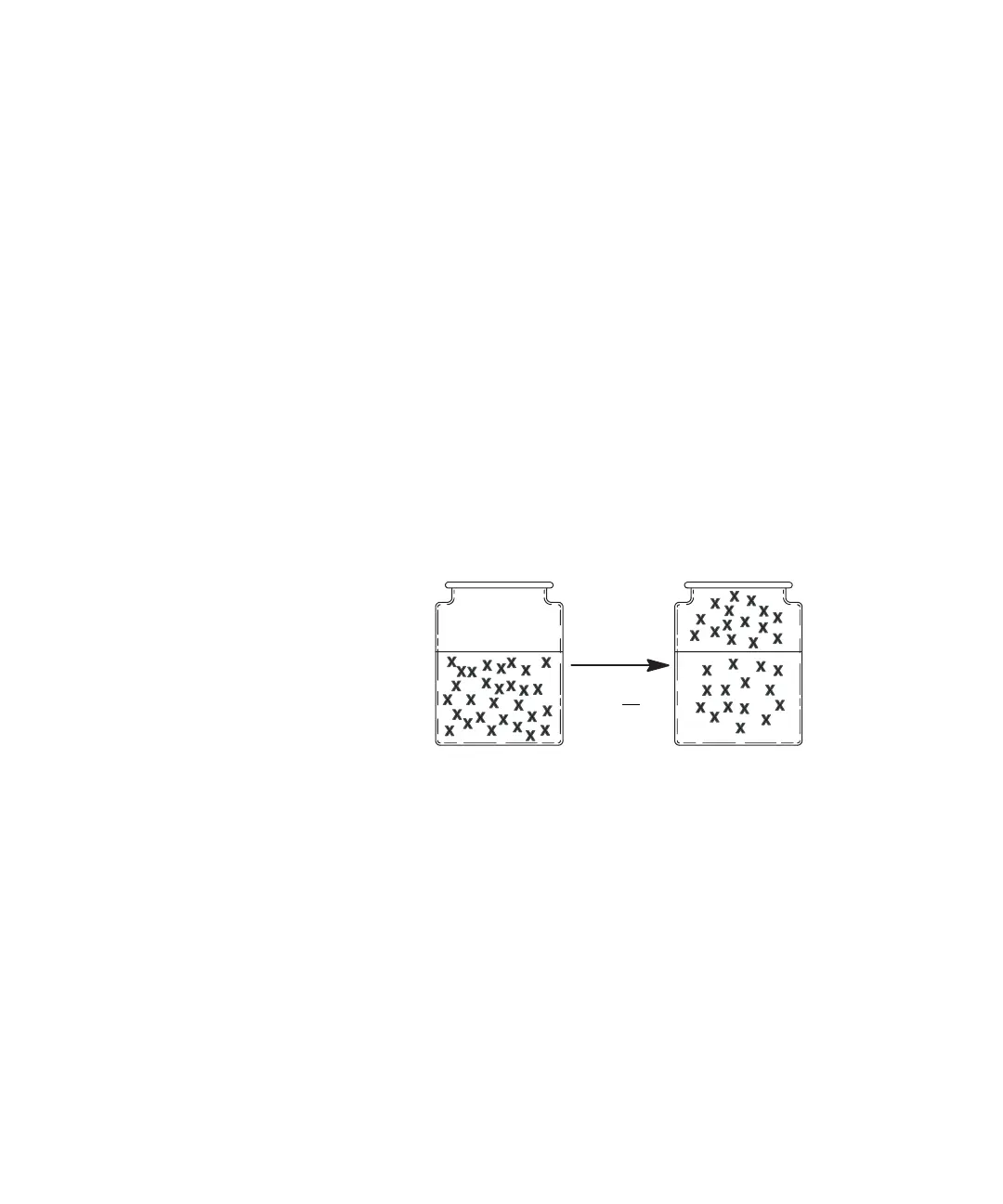
The tendency of a material to go into the gaseous phase is the
partition coefficient, K, where C
c
is the concentration of the
analyte in the condensed phase (the sample matrix) and C
g
is
the concentration of the analyte in the gaseous phase (the
headspace). The partition coefficient, K is related to the degree
of solubility that the analyte has in the matrix. For example,
benzene is not very soluble in water, and has a K of
approximately seven. Ethanol, which is very soluble in water,
has a K of 7000. A high value of K implies it is difficult for the
analyte to leave the matrix and go into the headspace. See
Figure 15 for an illustration of the particle coefficient.
K is also very dependent on temperature, as demonstrated by
the following equation:
Figure 15 Illustration of partition coefficient
Equilibration
K =
C
c
C
g
dK
dT
--------
1
T
2
-------=
medium_standard.book Page 108 Tuesday, February 17, 2004 10:14 AM

 Loading...
Loading...











