MAN-02624-001 Rev. 003 page 5 of 15
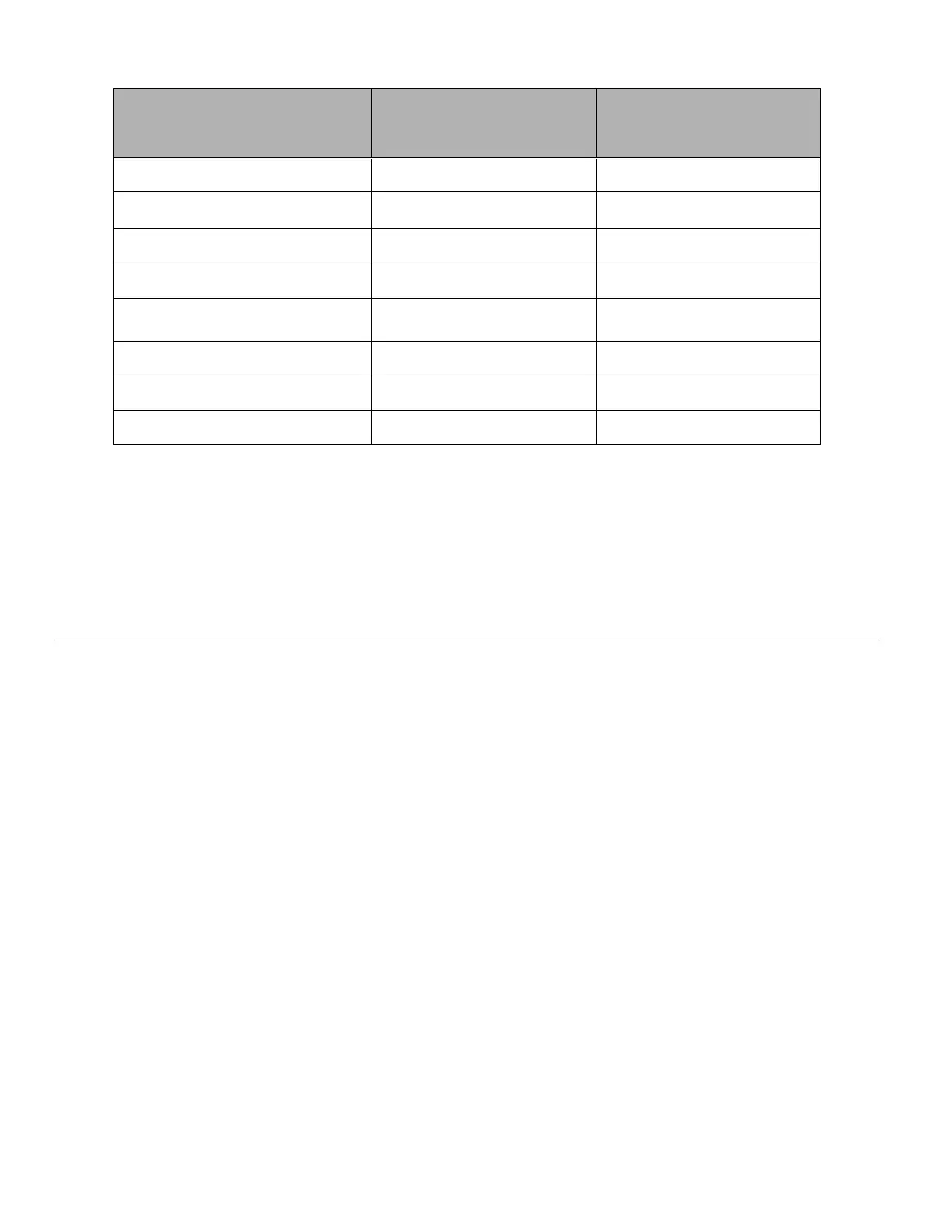
Organism Initial Concentration
Log Reduction after
15 min.
Candida albicans 5.5 x 10
5
CFU/mL >4.7
Aspergillus niger* 4.8 x 10
5
CFU/mL 2.7
Escherichia coli 2.8 x 10
5
CFU/mL >4.4
Staphylococcus aureus 2.3 x 10
5
CFU/mL >4.4
Pseudomonas aeruginosa 2.5 x 10
5
CFU/mL
>4.4
Mycobacterium tuberculosis** 9.4 x 10
5
CFU/mL 4.9
Rabbitpox virus 6.0 x 10
6
PFU/mL 5.5***
HIV-1 1.0 x 10
7.5
TCID
50
/mL 7.0***
* After 1 hour >4.7 log reduction
** After 1 hour >5.7 log reduction
*** Data is for 5 minutes
PERFORMANCE CHARACTERISTICS: REPORT OF CLINICAL
STUDIES
A prospective multi-center clinical study was conducted to evaluate the performance of the ThinPrep
2000 System in direct comparison to the conventional Pap smear. The objective of the ThinPrep
clinical study was to demonstrate that gynecologic specimens prepared using the ThinPrep 2000
System were at least as effective as conventional Pap smears for the detection of atypical cells and
cervical cancer or its precursor lesions in a variety of patient populations. In addition, an assessment of
specimen adequacy was performed.
The initial clinical study protocol was a blinded, split sample, matched pair study, for which a
conventional Pap smear was prepared first, and the remainder of the sample (the portion that normally
would have been discarded) was immersed and rinsed into a vial of PreservCyt Solution. At the
laboratory, the PreservCyt sample vial was placed into a ThinPrep 2000 Processor and a slide was then
prepared from the patient’s sample. ThinPrep and conventional Pap smear slides were examined and
diagnosed independently. Reporting forms containing patient history as well as a checklist of all
possible categories of The Bethesda System were used to record the results of the screening. A single
independent pathologist reviewed all discrepant and positive slides from all sites in a blinded fashion to
provide a further objective review of the results.
LABORATORY AND PATIENT CHARACTERISTICS
Cytology laboratories at three screening centers (designated as S1, S2, and S3) and three hospital
centers (designated as H1, H2, and H3) participated in the clinical study. The screening centers in the
study serve patient populations (screening populations) with rates of abnormality (Low-grade
Squamous Intraepithelial Lesion [LSIL] and more severe lesions) similar to the United States average
of less than 5%.
2
The hospital centers in the study serve a high risk referral patient population
(hospital populations) characterized by high rates (>10%) of cervical abnormality. Data on race

 Loading...
Loading...