SonixTouch Q+ Service Manual 00.053.204, Revision A Chapter 11: Maintenance
11-13
11.3.6 Cleaning/Disinfecting Endocavity Transducers
Endocavity transducers are semi-critical medical devices and must be decontaminated using, at a minimum, High
Level Disinfection.
Clean and disinfect transducers prior to the first exam and following each exam thereafter.
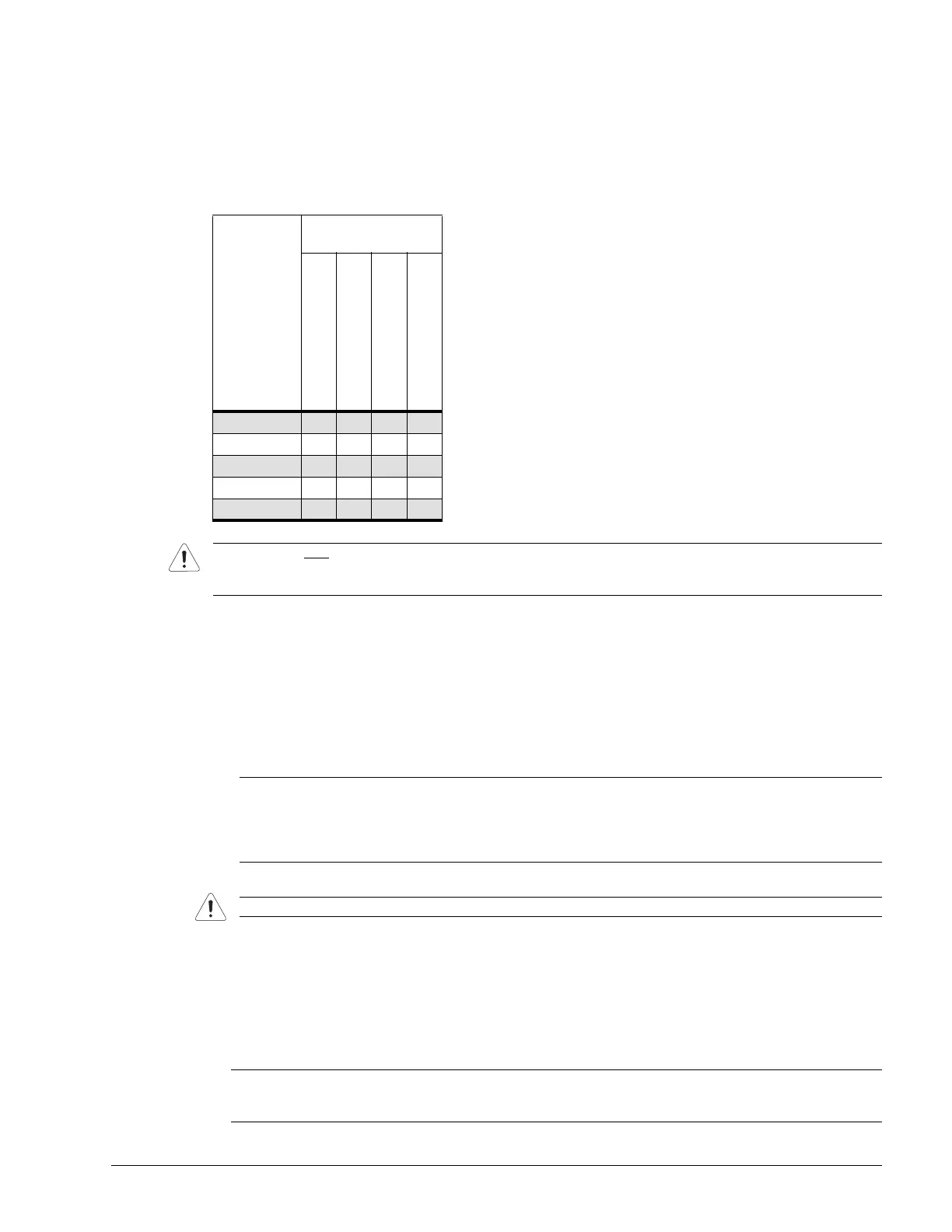
Table 11-4: Endocavity Cleaning/Disinfecting Agents
To Clean/Disinfect a Transducer:
1. Unplug the transducer.
2. Wash the transducer head and cable with soap and water to remove any protein buildup; however do not rinse
or immerse the transducer near the strain relief.
3. Following the manufacturer’s instructions, disinfect the transducer with a recommended disinfecting agent from
Table 11-4.
4. Wipe with a clean, dry cloth.
11.3.7 Sterilization
Sterilization of transducers is not possible. Follow the instructions for cleaning and disinfection instead:
• Endocavity transducers: 11.3.6
• Non-invasive transducers: 11.3.5.1.and 11.3.5.2.
ENDOCAVITY
T
RANSDUCERS
C
LEANING/DISINFECTING
A
GENTS
Cidex Activated Dialdehyde
Solution 14 day
Cidex Plus 28 day
Cidex OPA
Cidezyme
EC9-5/10
♦ ♦ ♦ ♦
EC9-5/10 GPS
♦♦♦♦
4DEC9-5/10
BPC8-4/10
BPL9-5/55
♦ ♦ ♦ ♦
Caution: Use only Ultrasonix recommended cleaners/disinfectants (Table 11-4). They have been tested and
determined safe to use on Ultrasonix transducers. Failure to follow these instructions may cause damage
and will void transducer warranties.
Note: Where any transducer (including, but not limited to, an intracavity transducer) is used in a clinical
application of a semi-critical nature (including, but not limited to intraoperative, transrectal, transvaginal,
transesophageal, etc.), ensure the transducer is covered with the appropriate STERILE transducer cover/
sheath which has received regulatory clearance for use. Refer to Third Party Accessories in Appendix B
of the relevant Extended User Manual for the recommended transducer cover/sheath.
Caution: Do not allow cleaning solutions to air dry on the transducer.
Note: Where transducers (non-critical and semi-critical medical devices/equipment) cannot withstand
sterilization, the FDA recognizes the use of a sterile gel and a sterile transducer cover as an acceptable
method of infection control for ultrasound transducers.
 Loading...
Loading...