4-8
P129394-176 Operator Manual Techniques of Sterilization
4.8 Recommendations
for Sterilizing Liquids
Important: Read the following paragraphs before sterilizing any
liquids in your sterilizer. The liquid cycle is for non-patient contact
use only.
Borosilicate glass is required because it is a superior glass capable
of resisting thermal shock. If glass not as thermally resistant is used,
a greater potential for bursting exists.
Vented closures are required because, by design, they release
internal pressure build-up by automatically venting the containers,
whereas pressure in unvented containers remains until the contents
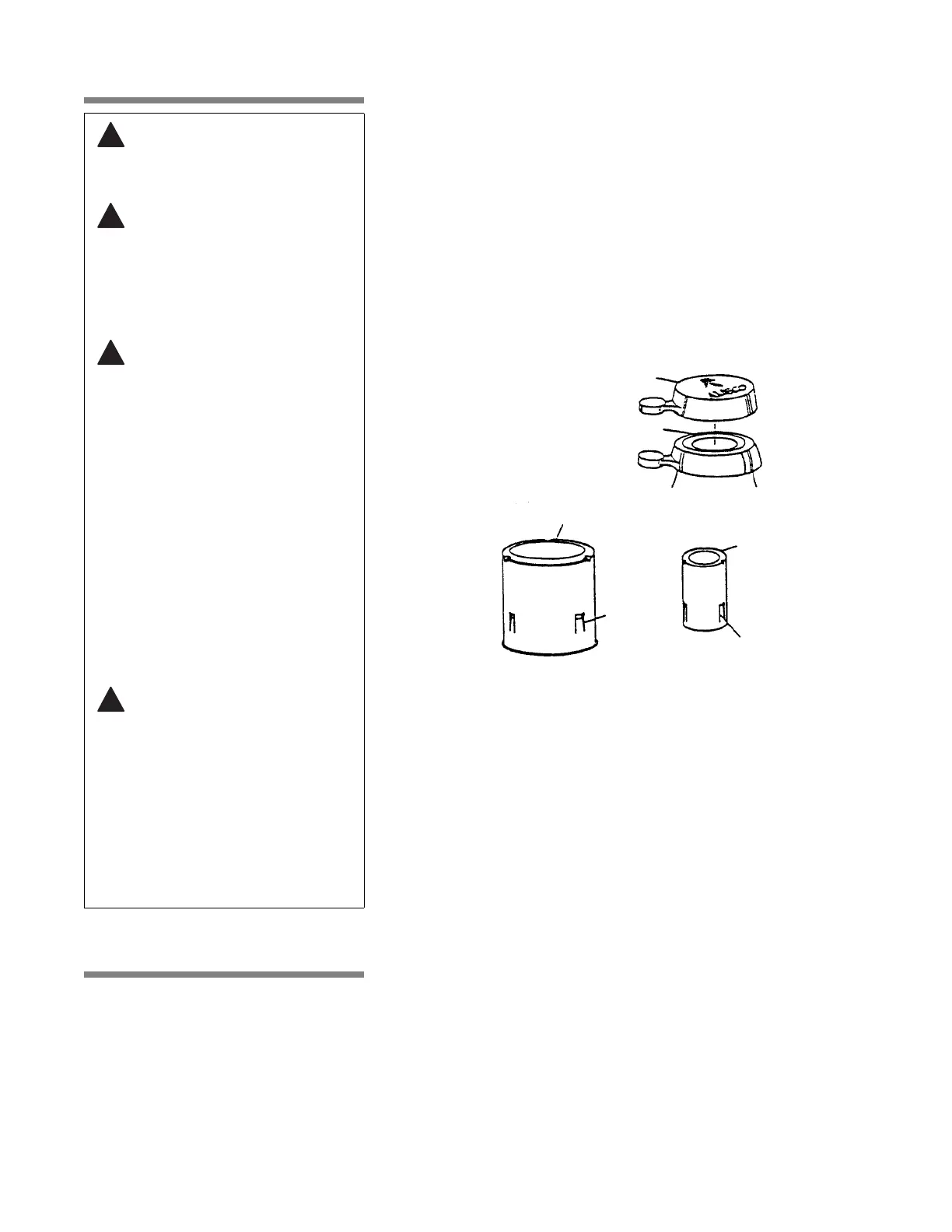
have cooled. Examples of vented closures are shown in Figure 4-1.
When loading, place small bottles in a separate basket to minimize
sliding. Always use side rails on the loading car to prevent
containers or baskets from falling off.
Figure 4-1. Vented Closures
4.9 Sterilization of
Implantable Devices
A healthcare facility must develop adequate monitoring practices for
the sterilization of implantable devices. Refer to AORN
recommended practices for more information.
Sterilization of implantable devices by a healthcare facility must be
monitored:
• When sterilizing an implantable device, always place a
biological monitor in the load.
• Following sterilization, implant must be quarantined until the
biological monitor has been incubated and read by a qualified
person.
WARNING – EXPLOSION
HAZARD: The liquid cycle is
for non-patient contact use
only.
WARNING – PERSONAL
INJURY HAZARD: Avoid
personal injury from bursting
bottles. Liquid sterilization
cycle must only be used for
liquids in borosilicate (Pyrex)
flasks with vented closures.
WARNING – BURN HAZARD:
When sterilizing liquids, you
must observe the following
procedures:
• Use Liquid cycle only.
• Use only vented closures.
• Use only Type I borosilicate
glass bottles.
• Do not allow hot bottles to be
jolted.
• Steam may be released
from the chamber when
door is opened. Step back
from the sterilizer each
time the door is opened to
minimize contact with
steam vapor.
CAUTION – POSSIBLE
EQUIPMENT DAMAGE:
Sterilization of chloride-
containing solutions (e.g.,
saline) can cause chamber
corrosion and is not
recommended by the
manufacturer. If, however,
chloride-containing
solutions must be
processed, clean the
chamber after each use.
Before Sterilization
After Sterilization
Morton Closure
Tab
Tab
Morton Closure
Morton Closure

 Loading...
Loading...


