F9, F9 Express Fetal & Maternal Monitor User Manual Safety Guidance
- 2 -
monitoring.
Contraindications:
They are not intended for use in intensive care units, operating rooms or for home use.
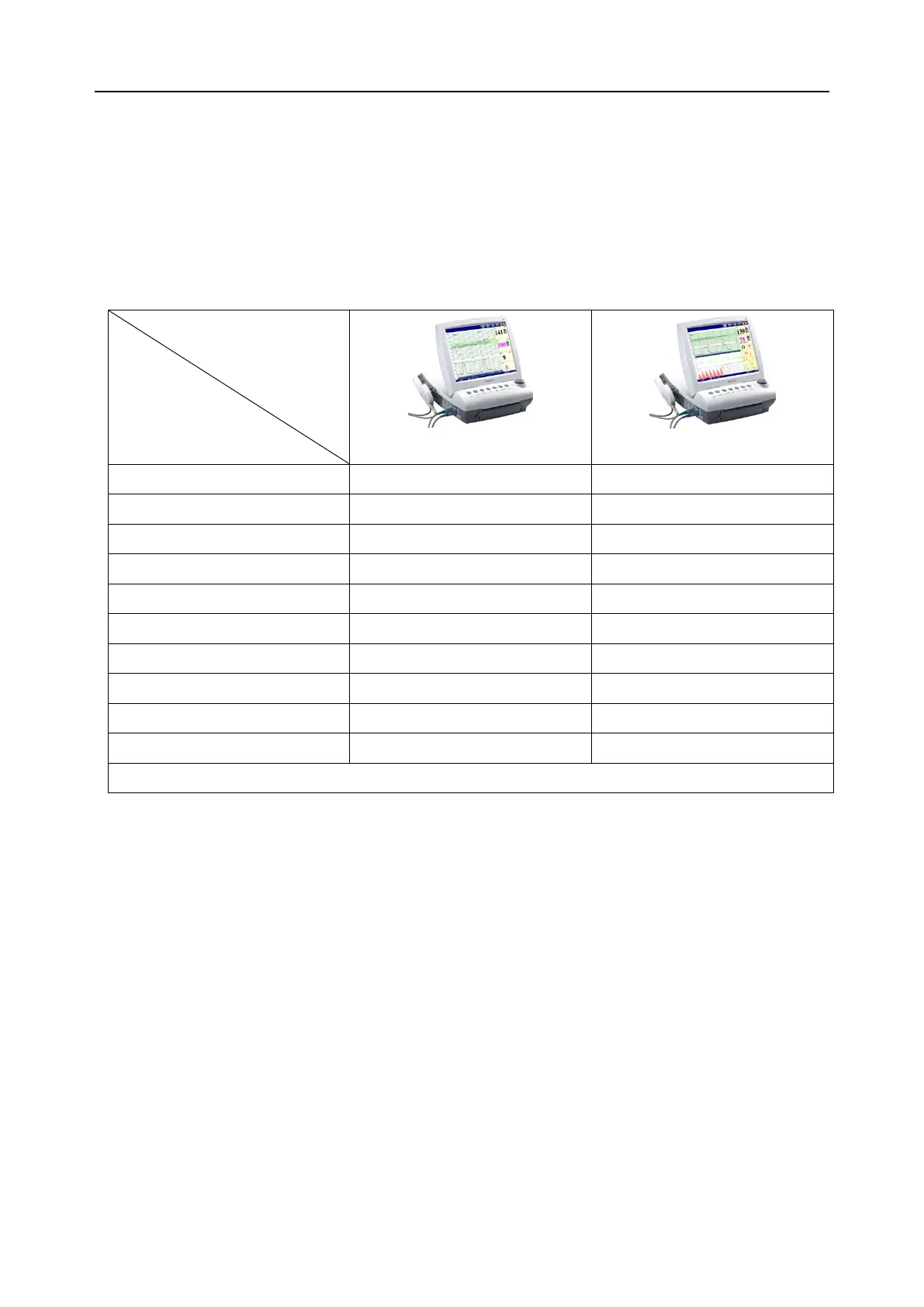
1.2 Features
The following table lists the measurements that F9 and F9 Express support.
NOTE: √ = Standard Opt = Optional × = Not Available
For the Essential Performance of F9 and F9 Express, refer to Appendix 1 in details.
1.3 Instruction for Safe Operation
NOTE:
In this manual, Monitor refers to both F9 and F9 Express, and is used where the
information applies to both models.
The monitor is designed to comply with the international safety requirements IEC/EN
60601-1 for medical electrical equipment. It is class I equipment.
The monitor operates within specifications at ambient temperatures between +5 ºC (+41 ºF)
and +40 ºC (+104 ºF). Ambient temperatures that exceed these limits could affect the
accuracy of the instrument and cause damage to the modules and circuits. Allow at least 2
inches (5 cm) clearance around the instrument for proper air circulation.
You must check that the equipment, cables and transducers do not have visible evidence of
damage that may affect patient safety or monitoring capability before each use. If damage is
evident, replacement is recommended before use.
 Loading...
Loading...