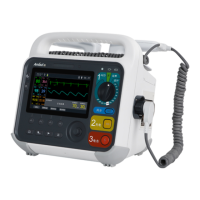
2 Product Introduction
2.1 Scope of application
The defibrillator monitori6 provides semi-automatedmated external defibrillation, manual
asynchronous external defibrillation, externally synchronized cardioversion, and non-invasive
external pacing for adults and children (including infants) and it also offers ECG, NIBP, SpO
2,
and
ETCO
2
monitoring for patients.
Semi-automated external defibrillation is for patients with suspected cardiac arrest who are
unresponsive, breathless, and pulseless; manual asynchronous external defibrillation is used to
terminate symptoms of tachycardia and ventricular fibrillation; externally synchronized
cardioversion is used to terminate atrial fibrillation; external non-invasive pacing is used in
patients with symptomatic bradycardia.
2.2 User Qualification
The defibrillator monitori6 is intended for use by personnel authorized by a doctor or chief
physician who have at least the following training and appropriate skills:
• Training on the use of defibrillator monitor i6.
• Trained in CPR or other physician-authorized emergency response plans.
• The Defibrillator Monitor i6 can be used in a medical institution or public places and
supports pre-hospital or in-hospital use.
• When used as a semi-automated external defibrillator in AED mode, the i6 is intended
for use by medical personnel trained in basic life support (including the use of AEDs).
• The i6 is intended for use by healthcare professionals trained in advanced life support
protocols when operating in monitoring, manual defibrillation, or pacer mode.
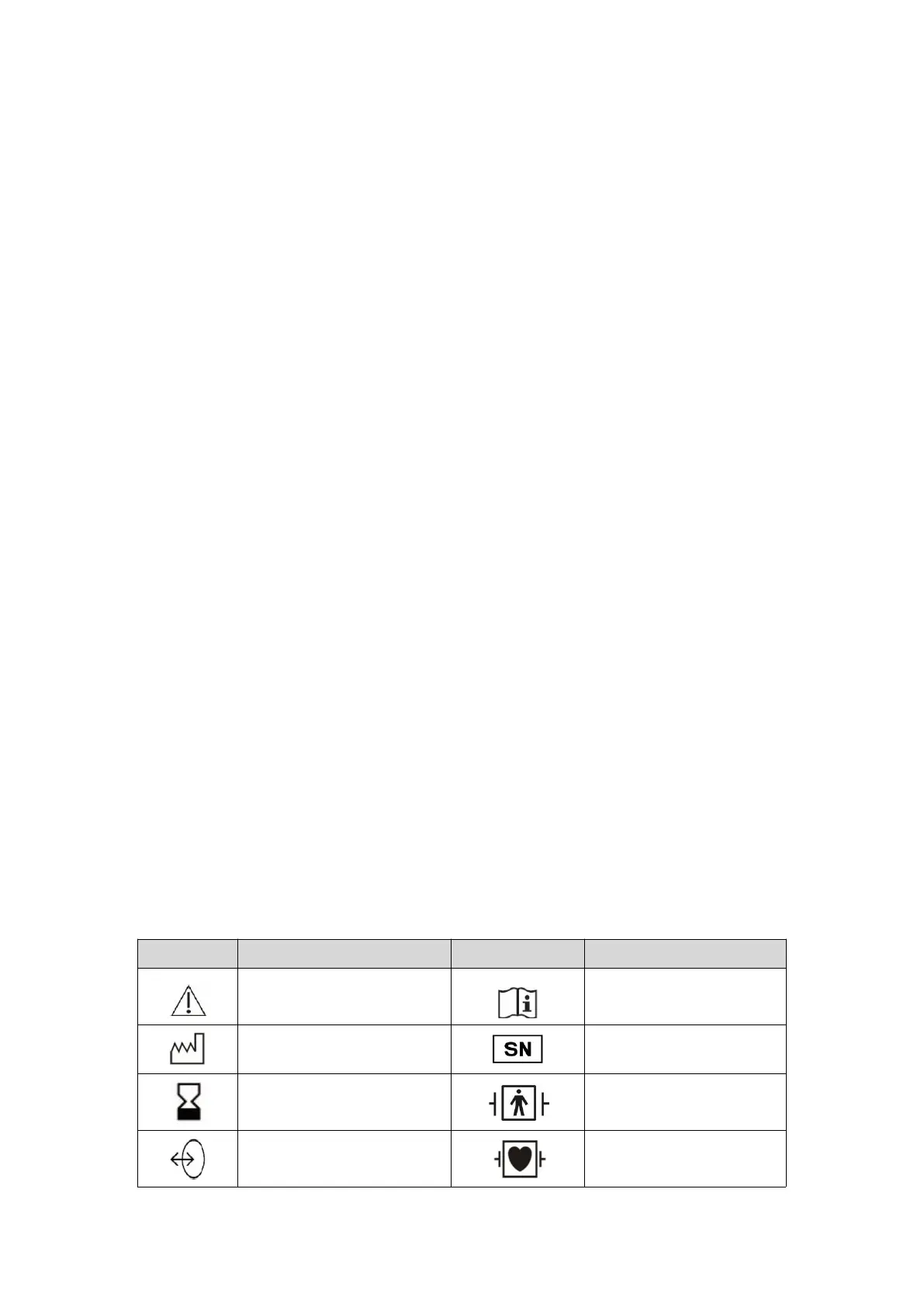
2.3 Symbols Used on the Equipment
The following table describes the symbols used on this device or in this Manual.
Refer to the documents
provided
Refer to the Instructions for
Use
The effective date for use
Anti-defibrillation BF type
application section
Anti-defibrillation CF type
application section
 Loading...
Loading...