9 - Regulatory Compliance
104 / 112
000HZ6006EN.US • 02 • GEM Hemochron 100 Operator Manual
RFID/Interference:
This instrument was tested and found compliant with RFID standard AIM 7351731 Rev 2:00: 2017‑02‑03.
Leakage Current:
This instrument was tested and found compliant with IEC 60601‑1:2005 +A1:2012, section 8.7.
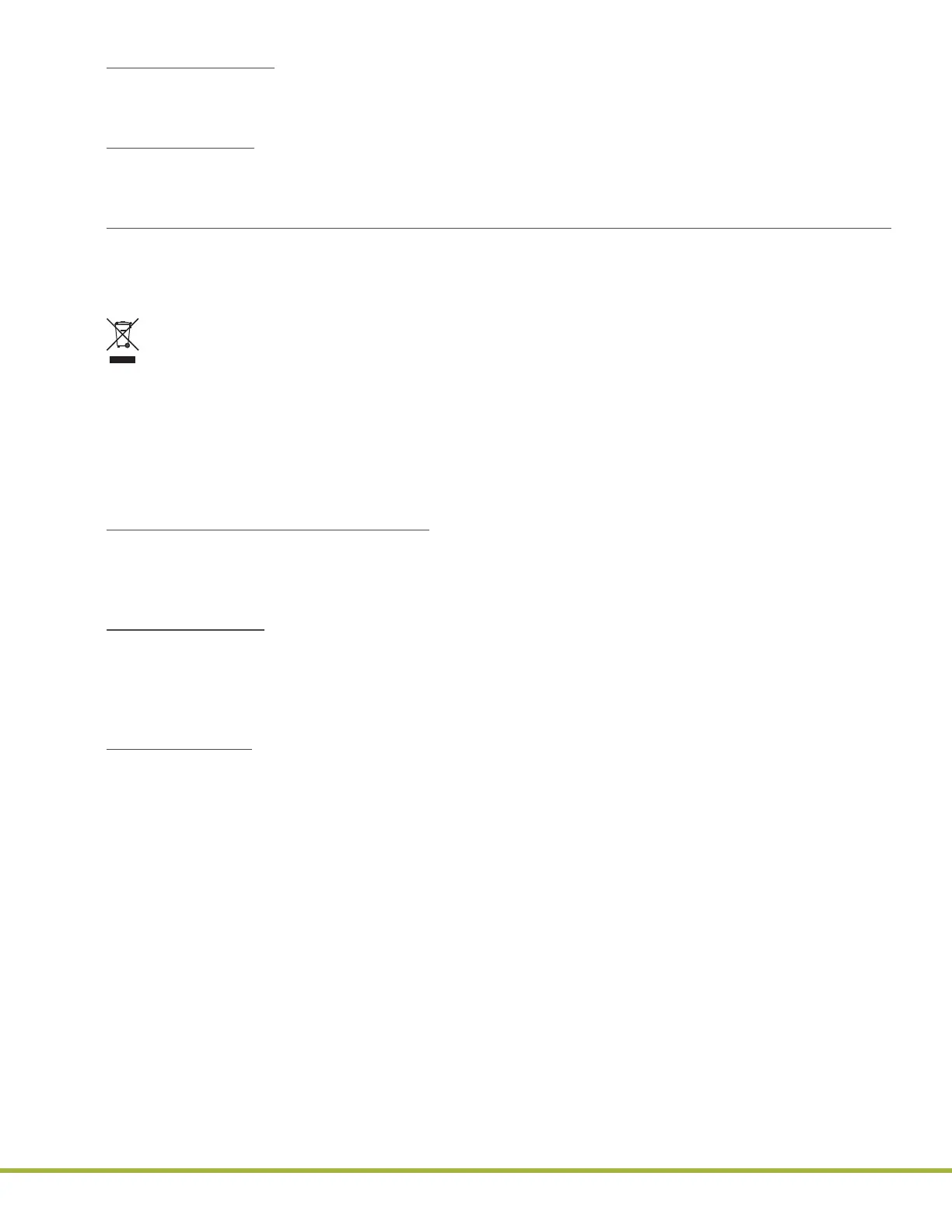
European Union Directive 2012/19/EU on Waste Electrical and Electrical Equipment(WEEE):
Instrumentation Laboratory is committed to meeting or exceeding the conditions of the WEEE Directive and
being a good environmental partner.
In compliance with the WEEE Directive, beginning with product shipped after August 13, 2005, all instruments
are labeled with the following symbol:
Disposing of this product correctly helps prevent potential negative consequences for the environment and for
human health. Recycling conserves natural resources.
Penalties may be applicable for incorrect disposal of this waste in accordance with national (European)
legislation.
Please contact your local distributor for information regarding the disposal of any end-of-life instruments.
Summary of Safety and Performance:
The Summary of Safety and Performance will be accessible in the European database on medical devices
(Eudamed) at https://ec.europa.eu/tools/eudamed. Currently, it is available on the manufacturer’s website.
RoHS Compliance:
The manufacturer certies that the GEM Hemochron 100 instrument is in compliance with the Directive
2011/65/EU of the European Parliament and of the Council of June 8, 2011 on the restriction of the use of
certain hazardous substances in electrical and electronic equipment (RoHS 3).
Electrical Safety:
Protection Against Ingress of Liquids: Ordinary (no protection as dened by IEC 60529).
Product Cleaning and Disinfection: Only according to recommendations of the manufacturer’s accompanying
documentation. Use PDI Super Sani to clean and disinfect the instrument. Bleach is not recommended.
Mode of Operation Of Equipment: Continuous
Degree of Safety of Application in the Presence of Flammable Anesthetic Mixture with Air, Oxygen, or Nitrous
Oxide: Not Suitable
NOTE: As dened in the above standards, the classication of Not Suitable is not intended to indicate that
the instrument is not suitable for use in an Operating Room (OR) environment. Rather, it is intended to
indicate that the instrument is not suitable for use in the direct presence of a ammable anesthetic mixture
with air, oxygen, or nitrous oxide.
All relevant documentation is kept on le at the manufacturer.

 Loading...
Loading...