Running an Experiment
172 0112-0109 H
probenecid can be useful in slowing dye leakage from cells, it is toxic to
cells, and therefore inclusion and duration of dye loading step should be
kept to a minimum.
Calcium Assay Kit Experimental Protocol
Cell Handling
The FLIPR
®
Calcium Assay Kit is designed to work with many cell types,
both adherent and non-adherent. We recognize that a variety of cell
handling conditions might be adopted at the discretion of the user,
based on standard operating procedures in the laboratory. In this
section we provide guidelines on how to prepare the cells for use with
the assay kit.
Adherent cells are the most frequently used cells with the kits. They are
typically plated the day prior to an experiment and then incubated in a
5% CO
2
, 37 °C incubator overnight. See the table below for suggested
plating volumes and seeding densities to create an 80–90% confluent
cell monolayer before placing the plates in the FLIPR
®
Tetra
Instrument.
For non-adherent cells, we recommend centrifuging cells from culture
medium and re-suspending the pellet in culture medium on the day of
the experiment. It is recommended after the cells are plated to
centrifuge the plates at 100g for up to 4 minutes (with brake off).
Alternatively, non-adherent cells can be treated like adherent cells,
plating the day before the assay using the same plating volumes and
seeding densities, as long as the cells are seeded onto coated plates
(for example, poly-D-lysine or collagen) to ensure good attachment to
the plate bottom.
Preparation of Loading Buffer
The following procedure is designed for preparation of the Loading
Buffer per vial of the Explorer Kit, the Bulk Kit, or the Express Kit.
1. To prepare the 1X HBSS Buffer plus 20 mM HEPES for the Bulk
and Express Kits only, (Explorer Kit contains ready to use HBSS
buffer plus 20 mM HEPES pH 7.4 in Component B), pipette 100
mL of 10X Hank’s Balanced Salt Solution and 20 mL of 1M Hepes
buffer pH 7.4 into 880 mL cell culture treated water.
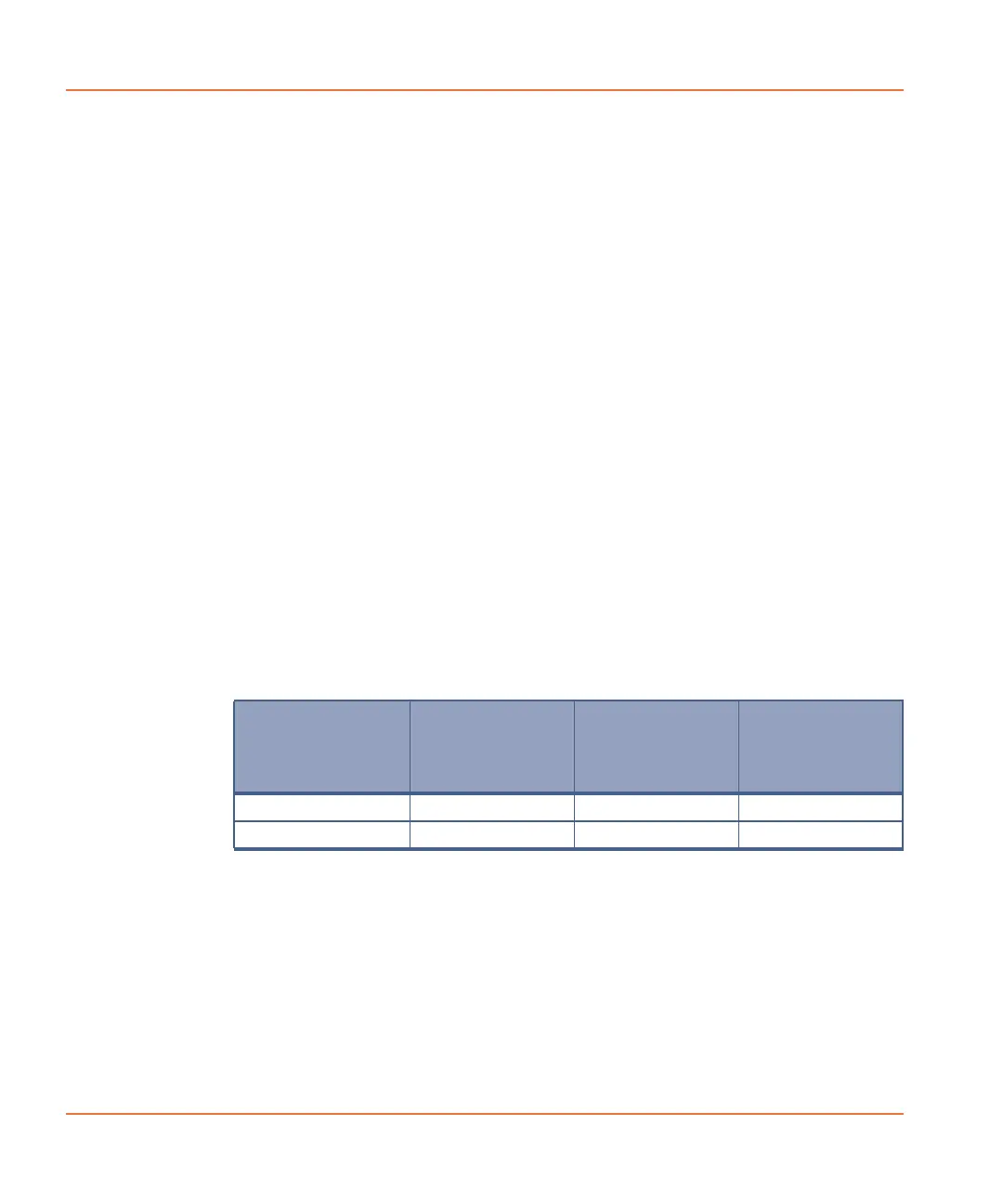
Cell Type
(cells/well)
96-well Plate
(100 μL
growth
medium)
384-well Plate
(25 μL growth
medium)
1536-well
Plate
(4 μL growth
medium)
Adherent cells 20,000–80,000 5,000–20,000 1,500–5,000
Non-adherent cells 40,000–200,000 10,000–50,000 3,000–10,000

 Loading...
Loading...