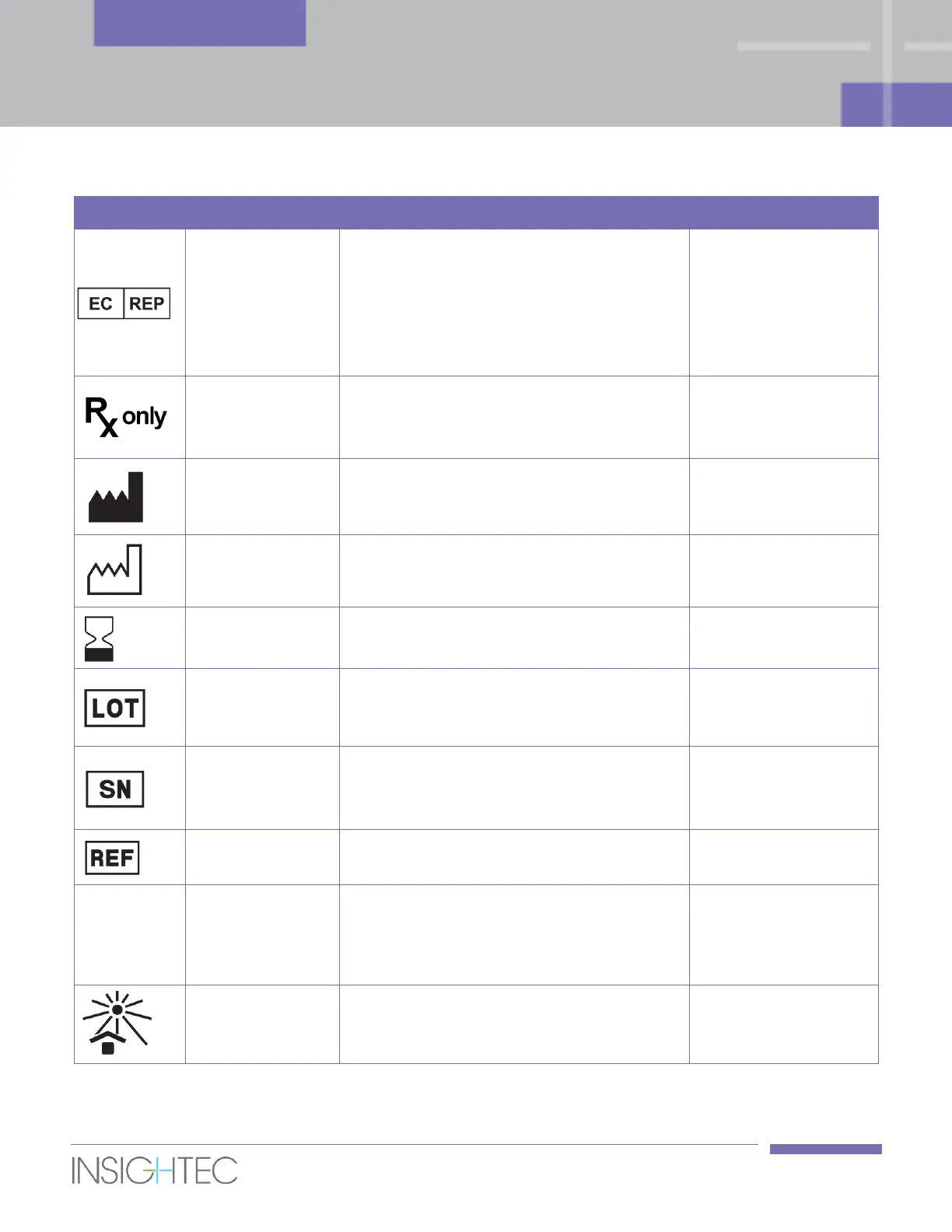
SAFETY
Manual Part Number
Authorized
representative in
the European
Community
This symbol shall be accompanied by the
name and address of the authorized
representative in the European
Community.
ISO 15223-1: 2016,
clause 5.1.2
Caution: Federal law restricts this device to
sale by or on the order of a physician /
special practitioner.
21 CFR 801.109
21 CFR
801.15(c)(1)(i)F
This symbol shall be accompanied by the
name and address of the manufacturer.
ISO 15223-1: 2016,
clause 5.1.1
This symbol shall be accompanied by a date
to indicate the date of manufacture.
ISO 15223-1: 2016,
clause 5.1.3
This symbol shall be accompanied by a date
to indicate the expiration date.
ISO 15223-1: 2016,
clause 5.1.4
This symbol shall be accompanied by the
manufacturer’s batch code. The batch code
shall be adjacent to the symbol.
ISO 15223-1: 2016,
clause 5.15
This symbol shall be accompanied by the
manufacturer’s serial number.
ISO 15223-1: 2016,
clause 5.1.7
The manufacturer’s catalogue number shall
be adjacent to the symbol.
ISO 15223-1: 2016,
clause 5.1.6
The name and/or number used to
represent one medical device, or a family of
medical devices to group many variations
that have shared characteristics.
Keep away from
sunlight/ Keep
away from heat
Indicates a medical device that needs
protection from light sources.
ISO 15223-1: 2016,
clause 5.3.2
 Loading...
Loading...