M|1|^^^25OHD^04^F^1|51||25OHD^0952^209525602987^20120229|System
Liquid^72634^00162^20121209\Cuvettes^18121^02177^20120506|
TRIGB^70245^00049^20130203\TRIGA^70244^00200^20130203\Wash
S^72508^00322^20121104\Wash1^2\Wash2\Wash3\DSORB
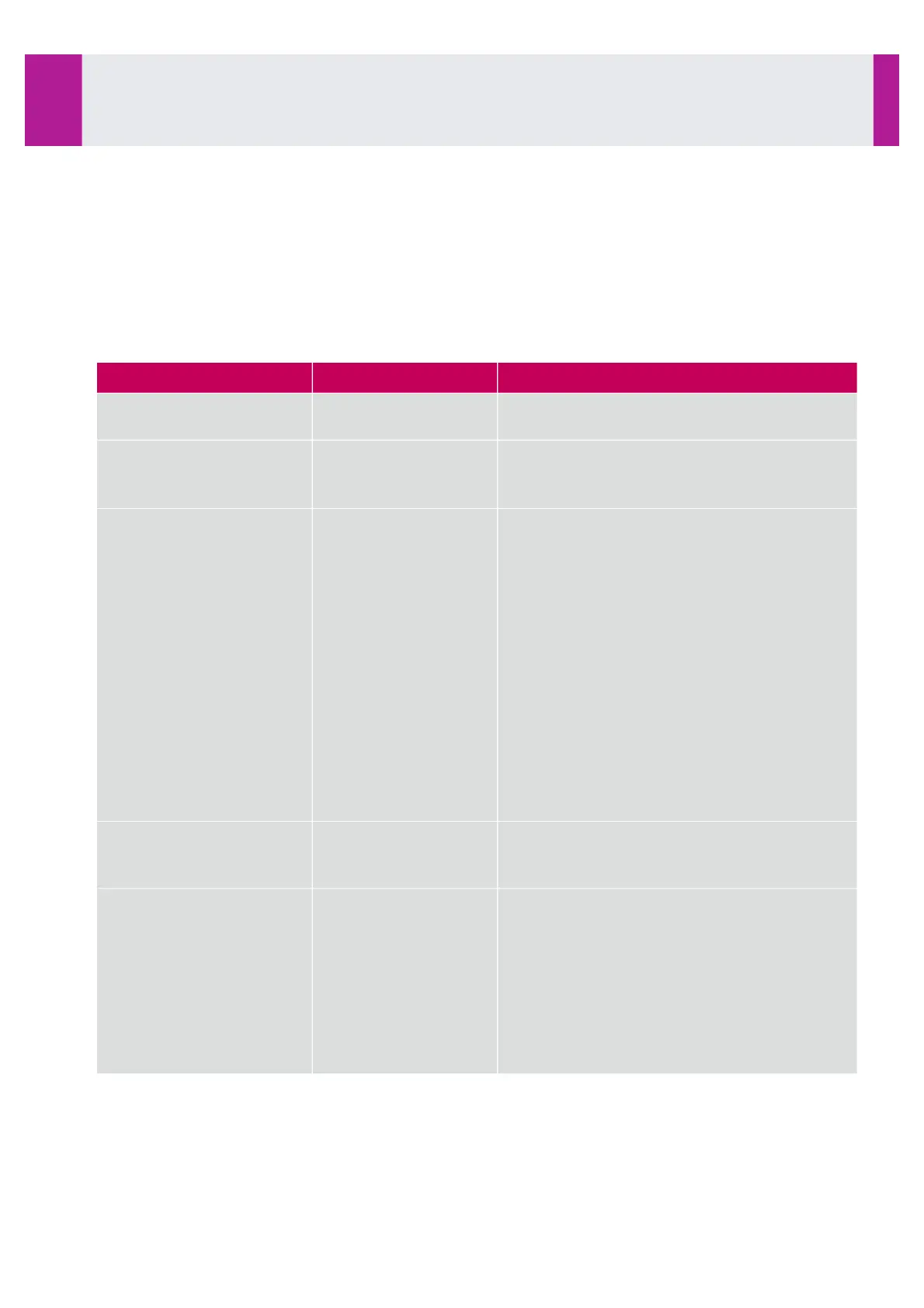
This block contains traceability information sent by IDS-iSYS to the LIS, and follows a Result block.
Here are the various fields of this block used in the protocol version:
Description and use in IDS-iSYS system
Fixed value M for indicating a Manufacturer
Information block.
Number of the block associated with a result,
begins at 1 and is increased for each
Manufacturer Information block in the message.
•
Universal test ID: NULL.
•
Universal test ID name: NULL.
•
Universal test ID type: NULL
•
Manufacturer’s code
•
Test type: 02 for a control, 04 for a patient.
•
Result type: always F for final.
Depending on the test, a final result can be a
mean value.
•
Replicate number: 1, 2, up to 10
•
Control identifier, defined as follow:
•
21 for a control Level 1
•
22 for a control Level 2
•
23 for a control Level 3
•
24 for a control Level 4
For a patient, this component is NULL.
•
Dilution rate: NULL if not applicable.
This field can take the values:
•
51 for Immunoassays,
•
52 for Biochemistry.
This field will be used only for control transfers.
For a patient, this field is NULL.
For a control, this field consists of:
•
target value,
•
low value of acceptable range,
•
high value of acceptable range
•
lot number
•
bottle number,
•
expiry date.
3- ASTM Compatible V2 Version
3-7- The Manufacturer Information Block
IDS-iSYS Connection Protocol - Revision N1"
3- ASTM Compatible V2 Version
" 30
Software version V 14
 Loading...
Loading...