5–14
Transpector CPM Operating Manual
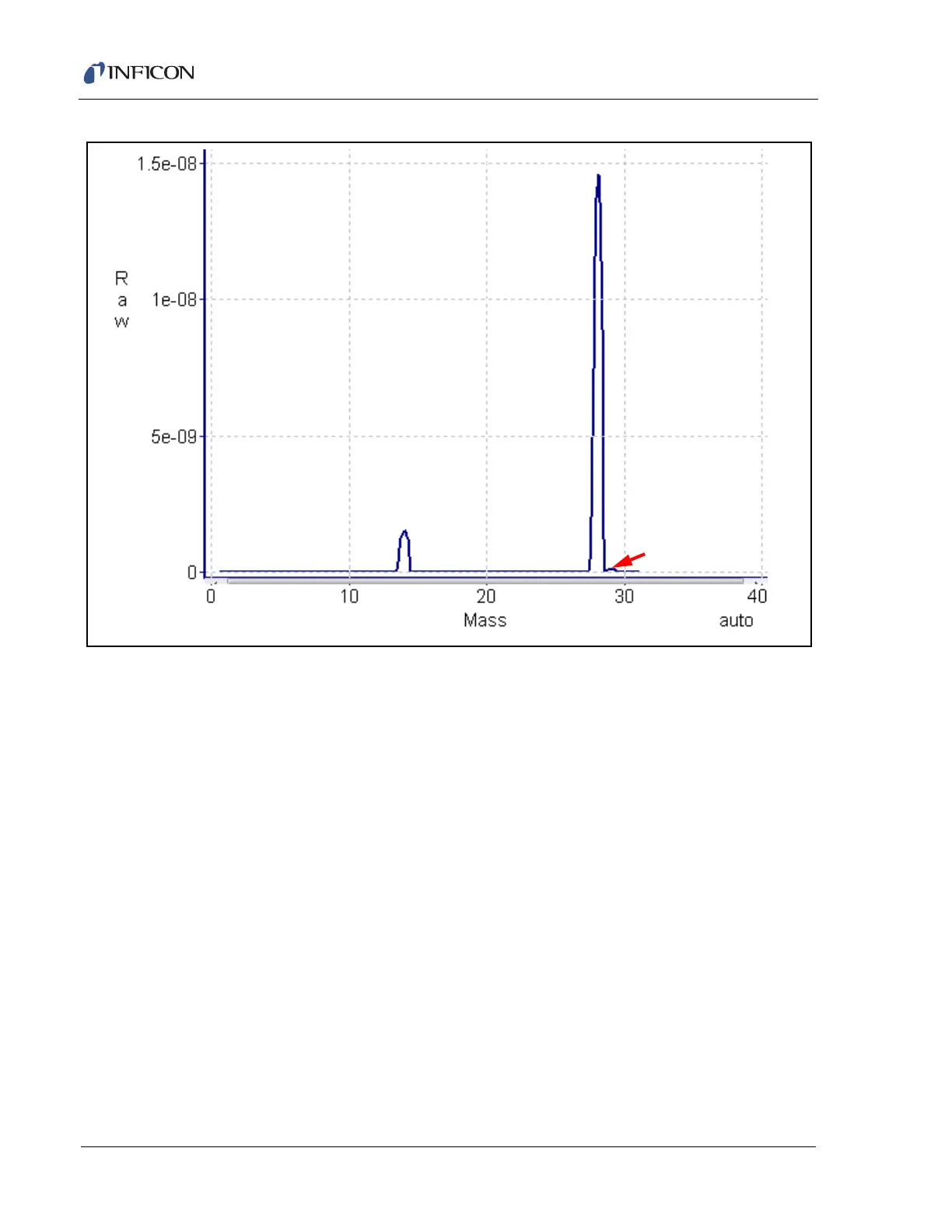
Figure 5-7 A nitrogen fragmentation pattern
This nitrogen fragmentation pattern shows
14
N
+
(14 AMU),
14
N
2
+
(28 AMU), and
14
N
15
N
+
(29 AMU).
In general, peaks from multiple charged species will be less intense than those for
the corresponding singly charged ion. For example, the doubly charged peak for
argon is typically less than one fifth as intense as the singly charged peak (this
intensity ratio is sensitive to the incident electron energy).
There are some situations when it is difficult to determine whether the ion is singly
or multiply charged. When a molecule is comprised of two atoms of the same
element, the typical partial pressure analyzer cannot distinguish between the singly
charged one-atom fragment ion and the doubly charged two-atom molecular ion,
which will both have the same mass-to-charge ratio. Refer to Figure 5-7. The peak
at 28 AMU is the parent ion, N
2
+
. It is not discernible from this spectrum if the peak
at 14 AMU is from N
+
or N
2
2+
. The 14 AMU peak in the nitrogen spectrum is from
the singly charged fragment ion.
Most ions (with the important exception of complex hydrocarbons) have masses
very close to integer values. When the mass of an ion is not evenly divisible by the
number of charges on it, the mass-to-charge ratio will not be an integer. Thus, Ar
3+
will appear at 13.33 AMU, while F
2+
will appear at 9.5 AMU.
 Loading...
Loading...