5–19
Transpector CPM Operating Manual
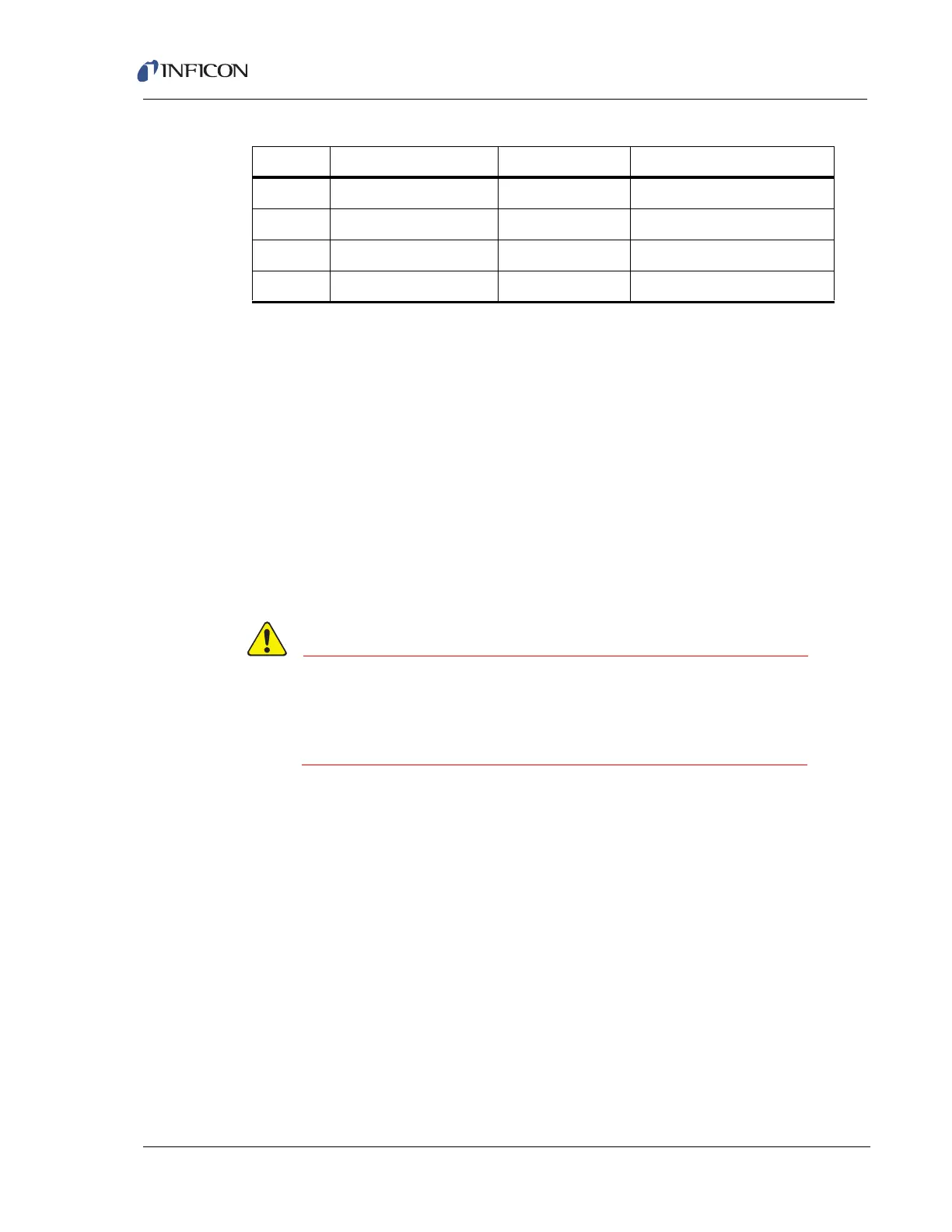
Table 5-3 shows that the appearance potential for Ar
2+
is 43.5 eV, while that for
H
2
O
+
is 13.5 eV. Therefore, by choosing an electron energy below 43.5 but above
13.5 eV, it is possible to produce the water vapor ion without producing doubly
charged argon ions, permitting the detection of water vapor at 18 AMU.
The Transpector CPM sensor and electronics can operate at electron energies
below 70 eV, with reduced electron emission (200 μA, max.). When monitoring
PVD processes, the CPM should be operated at 40 eV with an electron emission
current of 200 μA to reduce power to the filament. The software has the capability
to switch the CPM between 70 eV, 2.0 mA (CIS high) for background monitoring
and 40 eV, 200 μA (CIS low) for process monitoring.
With FabGuard Explorer, electron energy can be changed to a voltage ranging 10–
100 eV.
Below 70 eV it is necessary to limit the electron emission
current to no more than 200 μA, in order to not overpower
the filament. Overpowering the filament will result in
shortened filament life.
5.3.1.4 A Qualitative Interpretation Guide
A partial pressure analyzer identifies unknown substances by interpreting three
characteristics: fragmentation patterns, multiply charged ions, and isotope ratios.
Simple spectra are easy to interpret. The analysis of complicated mixtures is more
difficult.
Table 5-4 is a spectrum interpretation guide for examining an unknown spectrum.
It lists the masses of peaks, possible ion identities for each of these masses, and
common sources for each of these ions. This guide is by no means all-inclusive,
and only goes up to 50 AMU. Processes such as CVD and Etch often involve fairly
complex chemicals which provide very complicated spectra that extends to masses
well beyond 50 AMU.
H
2
O
+
water vapor 18 12.6
OH
+
water vapor 17 13.5
H
2
+
hydrogen 2 15.5
HF
+
hydrogen fluoride 20 16.1
Table 5-3 Appearance potentials for various ions from common gases
Ion Gas Mass-to-Charge Appearance Potential (eV)
 Loading...
Loading...