A-10
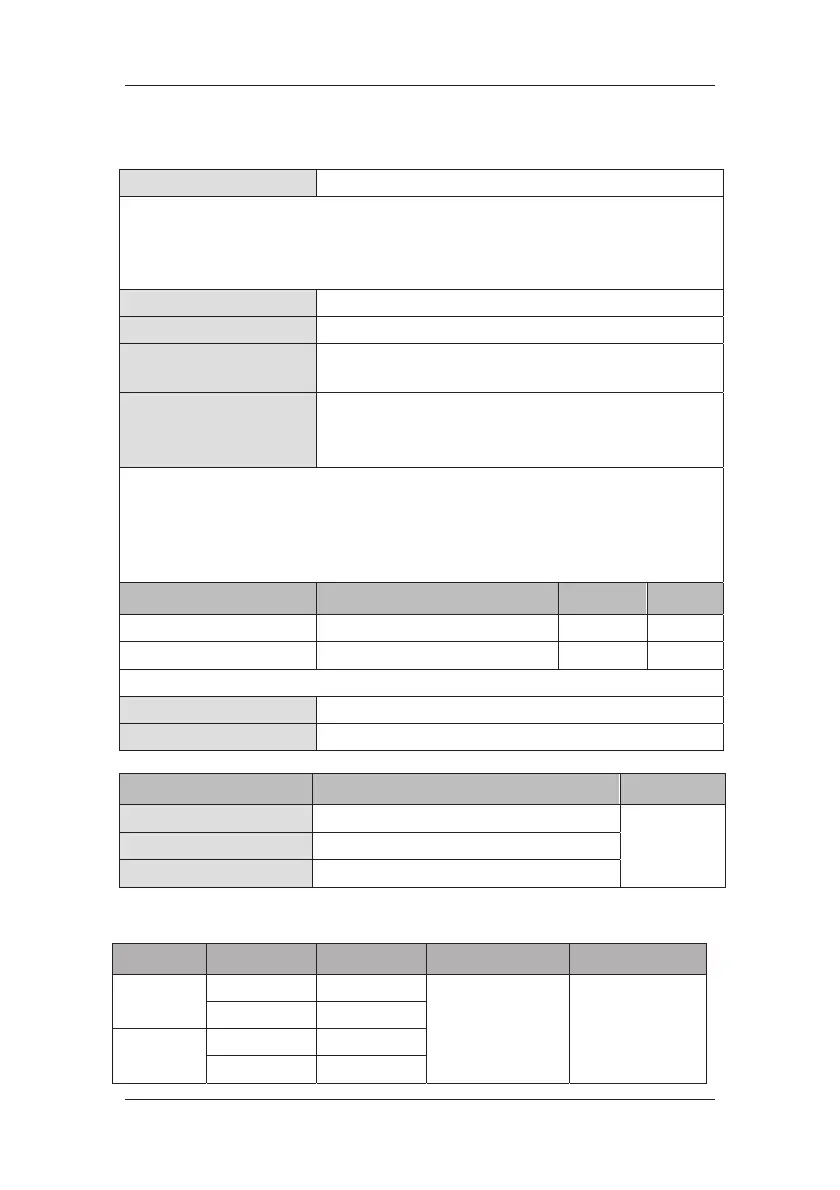
A.7.3 SpO
2
Standards Meet standards of ISO80601-2-61
*Measurement accuracy verification: The SpO
2
accuracy has been verified in human experiments
by comparing with arterial blood sample reference measured with a CO-oximeter. Pulse oximeter
measurement are statistically distributed and about two-thirds of the measurements are expected to
come within the specified accuracy range compared to CO-oximeter measurements.
SpO
2
measurement range 0 to 100%
Resolution 1%
Response time
30 s (PI > 0.3, no disturbance, SpO
2
value sudden change
within 70% - 100%)
Accuracy
70 to 100%: ±2% (adult/pediatric mode)
70 to 100%: ±3% (neonate mode)
0% to 69%: Not specified.
*Studies were performed to validate the accuracy of Pulse Oximeter with neonatal SpO
2
sensors by
contrast with a CO-Oximeter. Some neonates aged from 1 day to 30 days with a gestation age of
22 weeks to full term were involved in this study. The statistical analysis of data of this study
shows the accuracy (Arms) is within the stated accuracy specification. Please see the following
table.
Sensor type Totally neonates Data Arms
518B 97 (51 male & 46 female) 200 pairs 2.38%
520N 122 (65 male & 57 female) 200 pairs 2.88%
The Pulse Oximeter with neonatal SpO
2
sensors was also validated on adult subjects.
Refreshing rate 1 s
PI measurement range 0.05% to 20%
Alarm limit Range (%) Step (%)
SpO
2
High (low limit + 2) to 100
1
SpO
2
Low Desat to (high limit – 2)
Desat 0 to (high limit – 2)
Information of the Test Subjects of the Clinical Study Report:
Skin color Gender Number Age(years) Health
Black Male 1 28.2±9.19 Healthy
Female 1
yellow Male 3
Female 9
 Loading...
Loading...