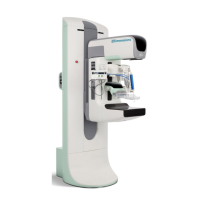
Brevera Breast Biopsy System User Guide
Chapter 2: General Information
MAN-04303-002 Revision 006 Page 11
Warning:
Control the access to the equipment according to local regulations for
radiation protection.
Warning:
This system can be dangerous to the patient and the user. Always follow the
safety precautions for x-ray exposures.
Warning:
As with any medical procedure, make sure that users wear appropriate
personal protective equipment to guard against potential contact with bodily
fluids.
Warning:
The Brevera breast biopsy system with CorLumina imaging technology is not
for use with MRI or Ultrasound.
Warning:
The Brevera system breast biopsy procedure should be performed only by
persons with sufficient training and familiarity with this procedure. Consult
medical literature relative to techniques, complications, and hazards before
performing any minimally invasive procedure.
Warning:
The Brevera biopsy device should only be used by physicians trained in
percutaneous biopsy procedures.
Warning:
Use sound professional judgment when using the Brevera breast biopsy
device on patients with breast implants.
Warning:
Minimally invasive instruments and accessories manufactured or distributed
by companies not authorized by Hologic may not be compatible with the
Brevera breast biopsy system. Use of such products can lead to unanticipated
results and possible injury to the user or patient.
Warning:
Instruments or devices that come into contact with bodily fluids can require
special disposal handling to prevent biological contamination.
 Loading...
Loading...