10
TITRATION THEORY
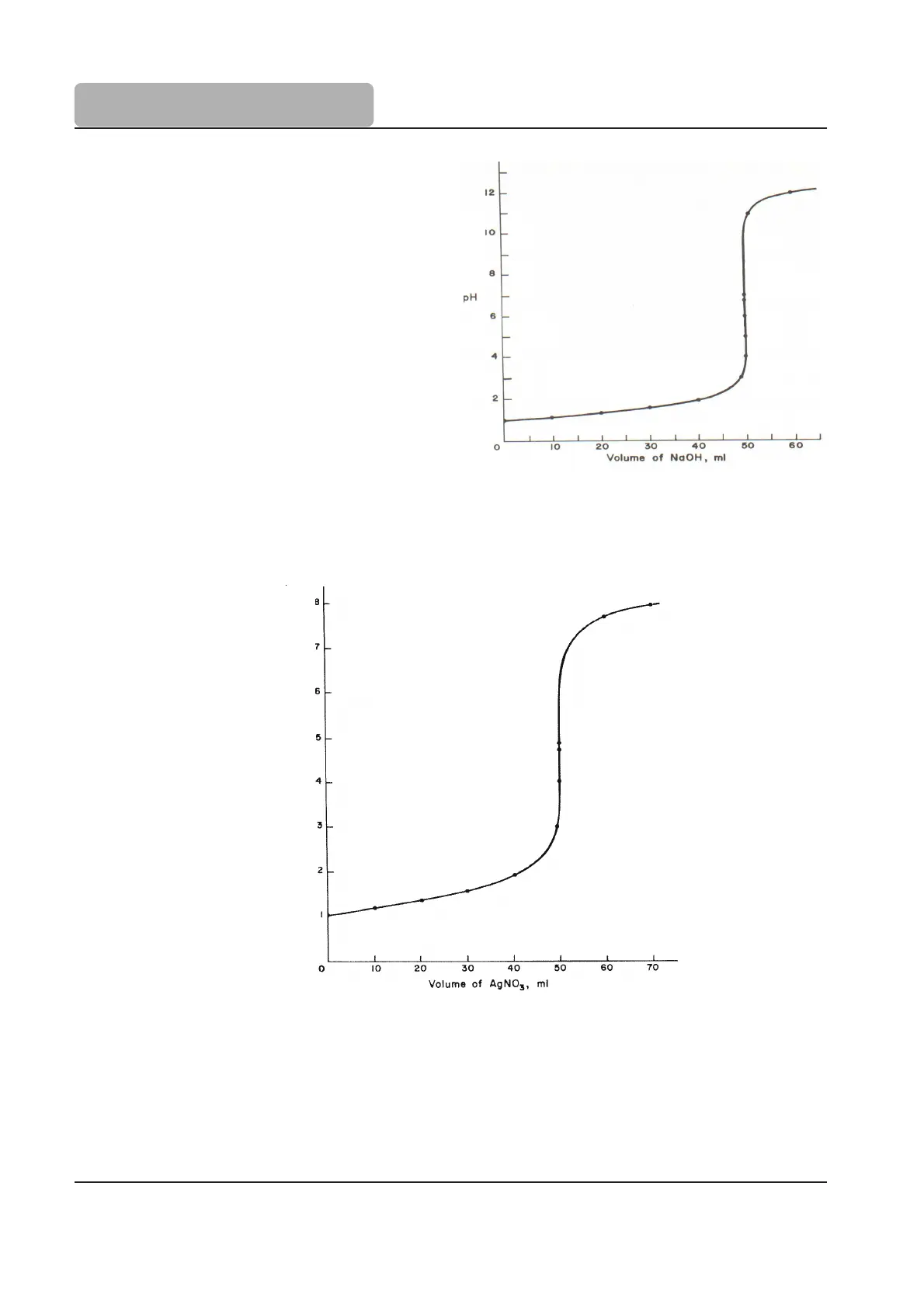
Figure 4
volume of NaOH added to an acidic solution
and the resulting pH of the solution. Note the
abrupt change in the pH at the equivalence point.
2.2.2 Argentometric Titrations
Argentometric titrations use silver (nitrate) as the
titrant and are generally precipitation titrations, as
many silver salts are insoluble. These titrations
are commonly used to titrate and determine the
concentration of bromide, chloride, cyanide,
iodide, and sulfide.
Argentometric titrations can be done with Mohr’s
indicator (when all of the chloride has reacted, a
red silver chromate precipitate is formed) or the titration can be easily followed with a silver ISE
(or chloride ISE for chloride titrations) and a reference electrode.
Figure 5 shows the titration of 50 mL of 0.1N NaCl with 0.1N AgNO
3
. The potentiometric
signal is from a chloride ISE and is plotted as pCl (- log [Cl
-
]).
2.2.3 Complexometric Titrations
A complex is a species where a central metal ion is covalently bonded to one or more electron
donating groups called ligands. In a complexometric titration, metal ions are titrated using a
titrant that binds strongly to it. Often these titrants contain EDTA or CDTA, polydentate ligands
that form very stable coordination compounds with metal ions. The complexation reaction
must be fast in order to be useful for direct titration. Some metal ions react too slowly with
EDTA for a direct titration.
Figure 5
 Loading...
Loading...