13
TITRATION THEORY
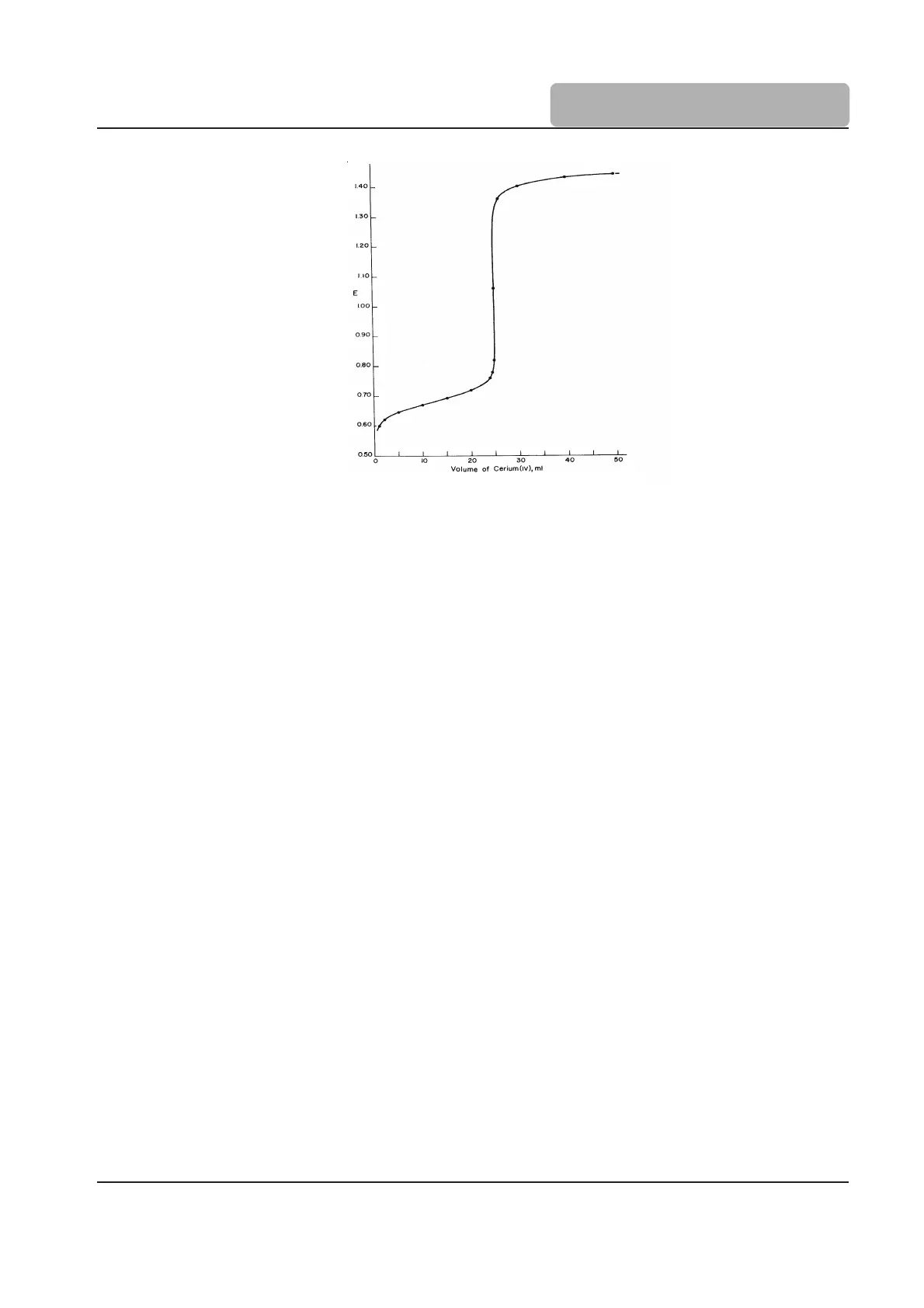
As with Acid-Base titrations the potential changes dramatically at the equivalence point.
2.2.8 Karl Fischer Titrations
This method is based on a well-defined chemical reaction between water and the Karl Fischer
reagent. The chemistry provides excellent specificity for water determination. The method
can be used to determine free and bound water in a sample matrix. The Karl Fischer method
is widely considered to produce the most rapid, accurate and reproducible results and has
the largest detectable concentration range spanning 1 ppm to 100%.
The determination of water content is one of the most commonly practiced methods in
laboratories around the world. Knowledge of water content is critical to understanding chemical
and physical properties of materials and ascertaining product quality. Water content
determination is conducted on many sample types including pharmaceuticals and cosmetics,
foods and natural products, organic and inorganic compounds, chemicals, solvents and
gases, petroleum and plastic products as well as paints and adhesives. The KF method is
verifiable and can be fully documented. As a result, Karl Fischer titration is the standard
method for analysis of water in a multitude of samples as specified by numerous organizations
including the Association of Official Analytical Chemists, the United States and European
Pharmacopoeia, ASTM, American Petroleum Institute, British Standards and DIN.
2.3 Titrations According to The Titration Sequence
2.3.1 Back Titrations
Back titrations are generally used when a reaction is too slow to be directly accomplished
using a “direct” titration, where the reaction goes to completion within a few seconds. In a
back titration, a large excess of a reagent is added to the sample solution, helping a slow
reaction to go to completion. The unreacted, excess reagent is then titrated. The difference
in the total volume of the first reagent added and amount determined from the second
titration is the quantity of reagent required to complete the first reaction.
Figure 8
 Loading...
Loading...