14
TITRATION THEORY
A
B
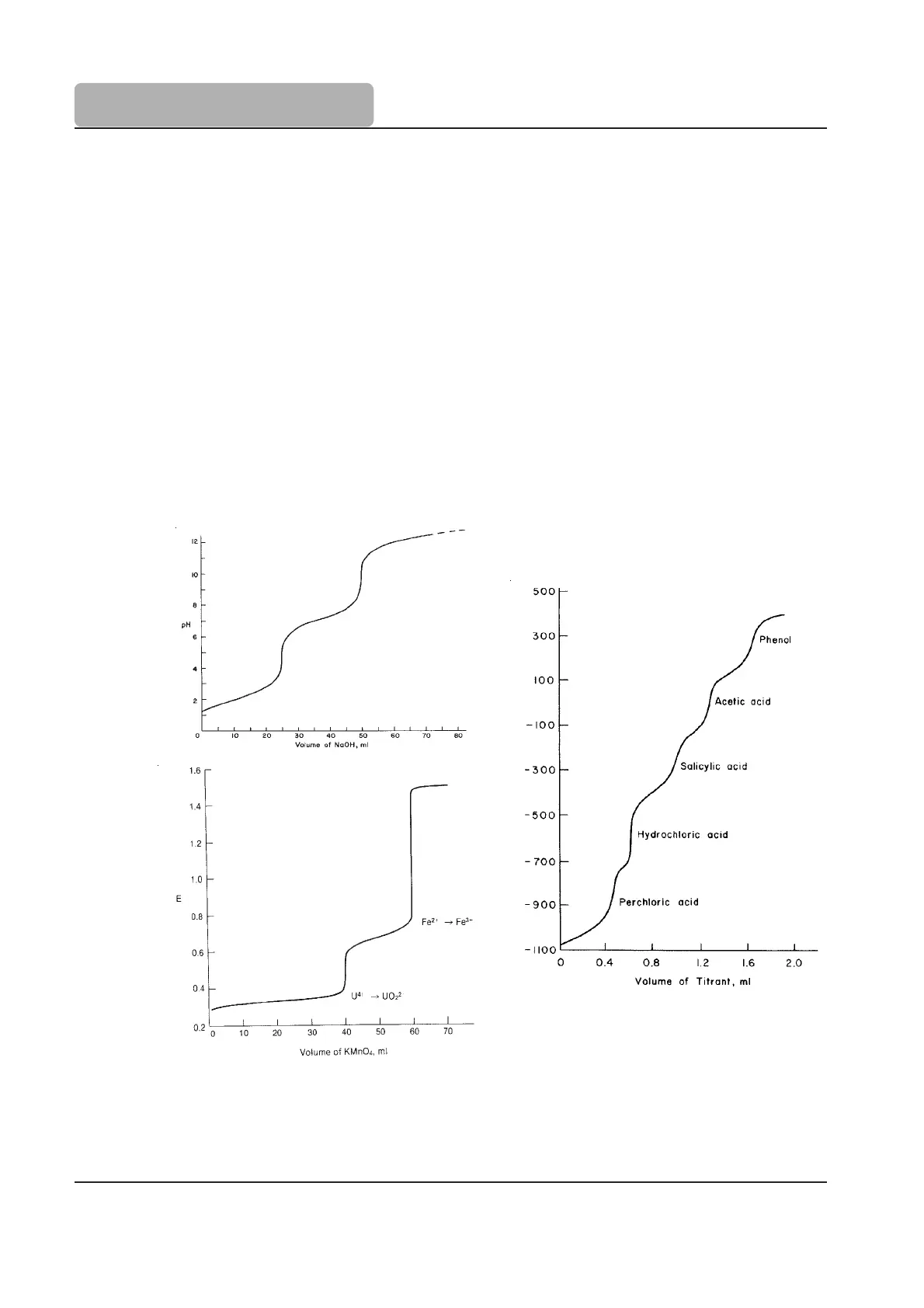
Figure 9
C
2.3.2 Multiple Endpoint Titrations
Under certain conditions, some titrations can exhibit more than one equivalence point and be
titratable to the individual endpoints to determine the concentration of each individual
component. Examples of these types of titrations include acid-base (where different strength
acid or bases are in a mixture), redox (where each species has a different reduction potential),
complexometric (where different species are separately titratable), and acid-base using
polyprotic acids (the pK
a
of the different protons varies enough to separate them).
Figure 9 shows three different types of multiple endpoint titrations. “A” shows the titration of
a polyprotic acid. The different acid strengths of the first and second proton can be determined.
“B” illustrates a mixture of two different metal redox species, where the different redox
potentials allow the species to be separated. “C” is the titration of a solution containing
strong, weak, and very weak acids.
 Loading...
Loading...