D Solvent Considerations
298 July 29, 2013, 715003794 Rev. B
• Temperature affects solvent miscibility. If you are running a
high-temperature application, consider the effect of the higher
temperature on solvent solubility.
• Buffers dissolved in water can precipitate when mixed with organic
solvents.
When you switch from a strong buffer to an organic solvent, flush the buffer
out of the system with distilled water before you add the organic solvent.
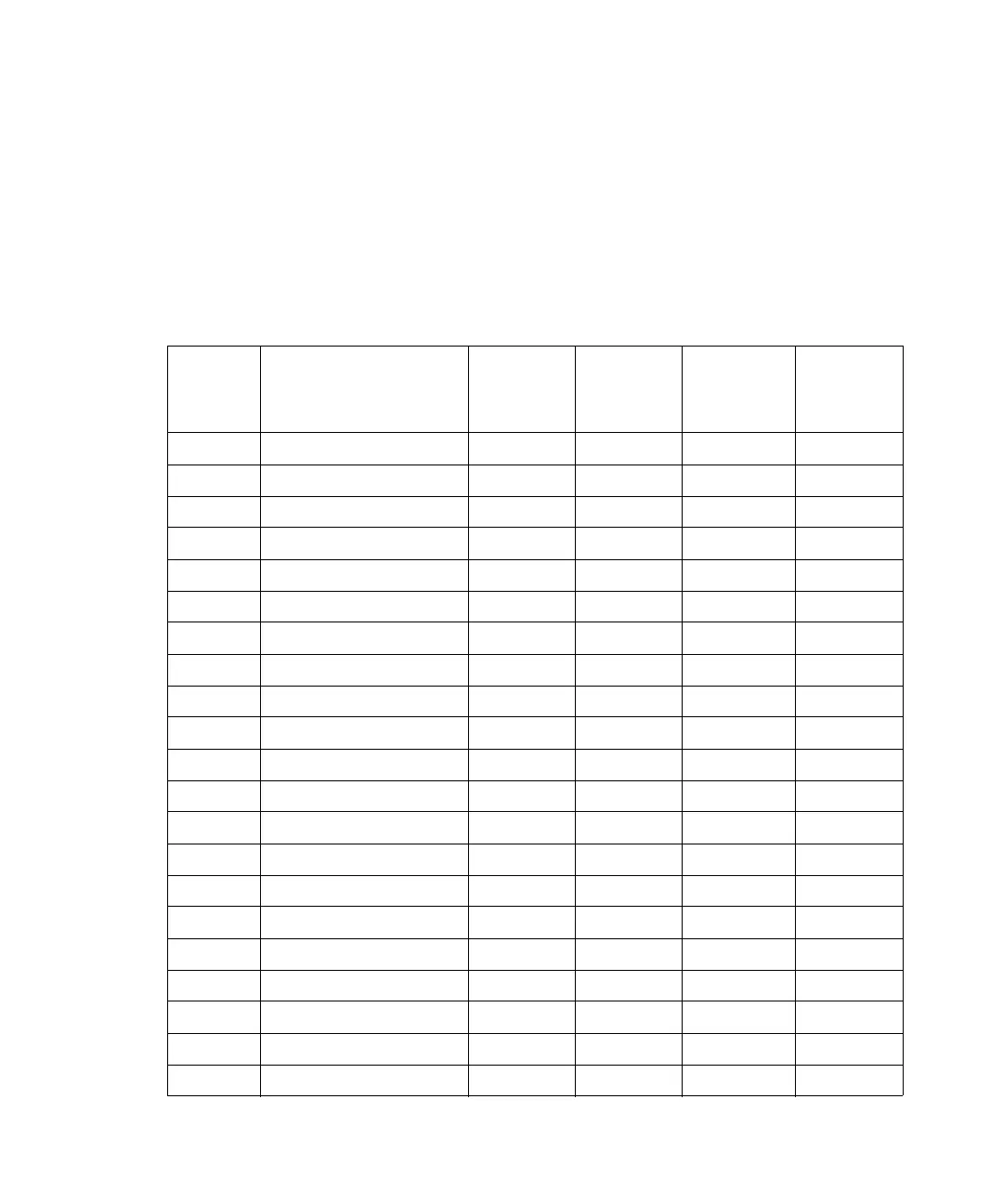
Solvent miscibility:
Polarity
index
Solvent
Viscosity
CP, 20 °C
Boiling
point °C
(@1 atm)
Miscibility
number
(M)
λ Cutoff
(nm)
–0.3 N-decane 0.92 174.1 29 ––
–0.4 Iso-octane 0.50 99.2 29 210
0.0 N-hexane 0.313 68.7 29 ––
0.0 Cyclohexane 0.98 80.7 28 210
1.7 Butyl ether 0.70 142.2 26 ––
1.8 Triethylamine 0.38 89.5 26 ––
2.2 Isopropyl ether 0.33 68.3 –– 220
2.3 Toluene 0.59 100.6 23 285
2.4 P-xylene 0.70 138.0 24 290
3.0 Benzene 0.65 80.1 21 280
3.3 Benzyl ether 5.33 288.3 –– ––
3.4 Methylene chloride 0.44 39.8 20 245
3.7 Ethylene chloride 0.79 83.5 20 ––
3.9 Butyl alcohol 3.00 117.7 –– ––
3.9 Butanol 3.01 177.7 15 ––
4.2 Tetrahydrofuran 0.55 66.0 17 220
4.3 Ethyl acetate 0.47 77.1 19 260
4.3 1-propanol 2.30 97.2 15 210
4.3 2-propanol 2.35 117.7 15 ––
4.4 Methyl acetate 0.45 56.3 15, 17 260
4.5 Methyl ethyl ketone 0.43 80.0 17 330
 Loading...
Loading...