Solvent miscibility
July 29, 2013, 715003794 Rev. B 299
How to use miscibility numbers (M-numbers)
Use miscibility numbers (M-numbers) to predict the miscibility of a liquid
with a standard solvent.
To predict the miscibility of two liquids, subtract the smaller M-number value
from the larger M-number value.
• If the difference between the two M-numbers is 15 or less, the two
liquids are miscible in all proportions at 15 °C.
• A difference of 16 indicates a critical solution temperature between
25 and 75 °C, with 50 °C as the optimal temperature.
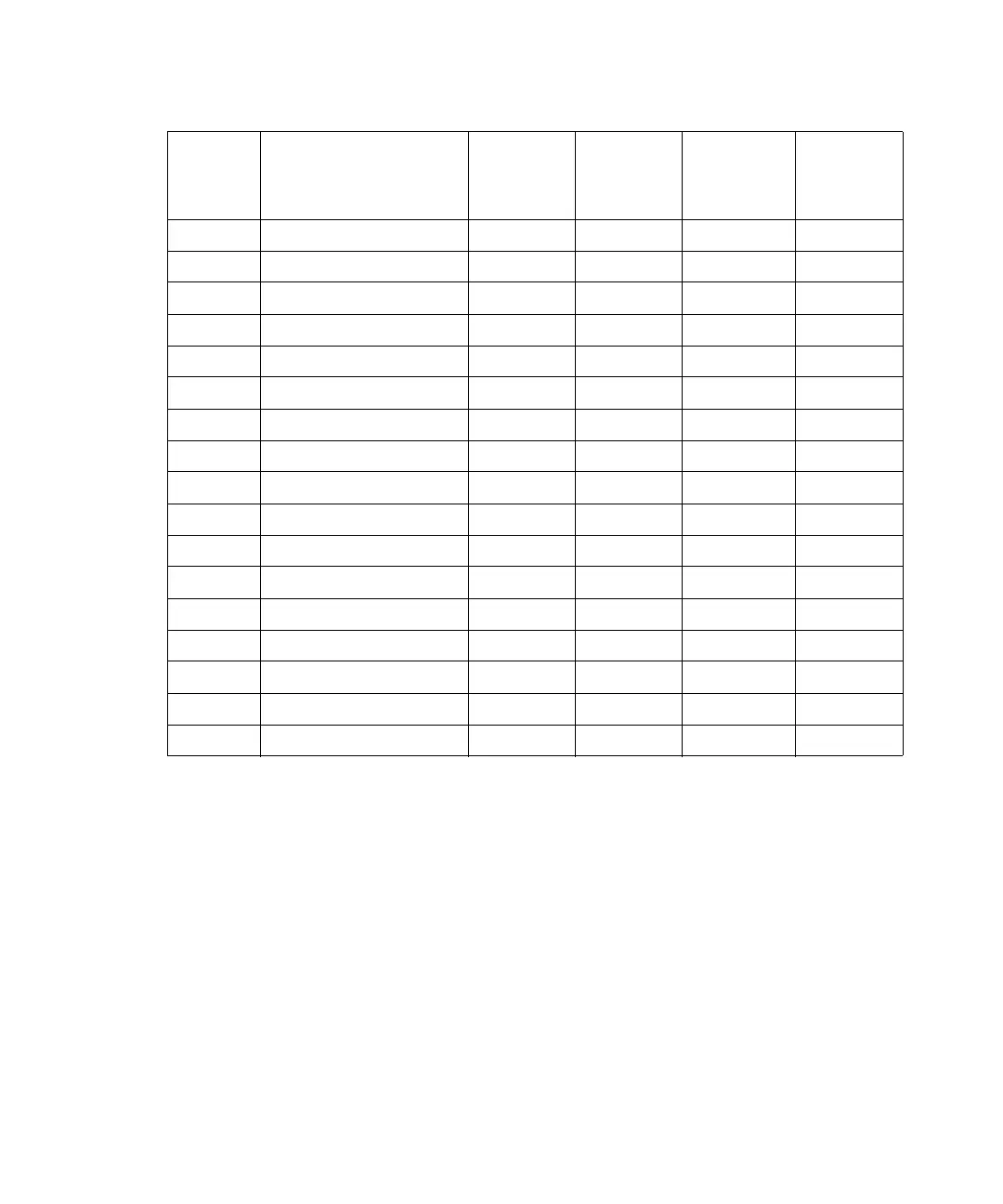
4.5 Cyclohexanone 2.24 155.7 28 210
4.5 Nitrobenzene 2.03 210.8 14, 20 ––
4.6 Benzonitrile 1.22 191.1 15, 19 ––
4.8 Dioxane 1.54 101.3 17 220
5.2 Ethanol 1.20 78.3 14 210
5.3 Pyridine 0.94 115.3 16 305
5.3 Nitroethane 0.68 114.0 –– ––
5.4 Acetone 0.32 56.3 15, 17 330
5.5 Benzyl alcohol 5.80 205.5 13 ––
5.7 Methoxyethanol 1.72 124.6 13 ––
6.2 Acetonitrile 0.37 81.6 11, 17 190
6.2 Acetic acid 1.26 117.9 14 ––
6.4 Dimethylformamide 0.90 153.0 12 ––
6.5 Dimethylsulfoxide 2.24 189.0 9 ––
6.6 Methanol 0.60 64.7 12 210
7.3 Formamide 3.76 210.5 3 ––
9.0 Water 1.00 100.0 –– ––
Solvent miscibility: (Continued)
Polarity
index
Solvent
Viscosity
CP, 20 °C
Boiling
point °C
(@1 atm)
Miscibility
number
(M)
λ Cutoff
(nm)
 Loading...
Loading...