360 EN MEDUCORE Standard
2
WM 68401 04/2021
17 Scope of supply
17.2 Accessories and other parts
This sub-section describes accessories and other parts in
accordance with the Medical Device Regulation (MDR). Accessories
are marked with a UDI-DI. Other parts do not have a UDI-DI. For
parts made by other manufacturers (third-party products) you can
request the UDI-DI from the manufacturer.
SD card, 32 GB – – WM 39510
Function test resistor – – WM 45428
MEDUCORE Standard
2
instructions for use
– – WM 68401
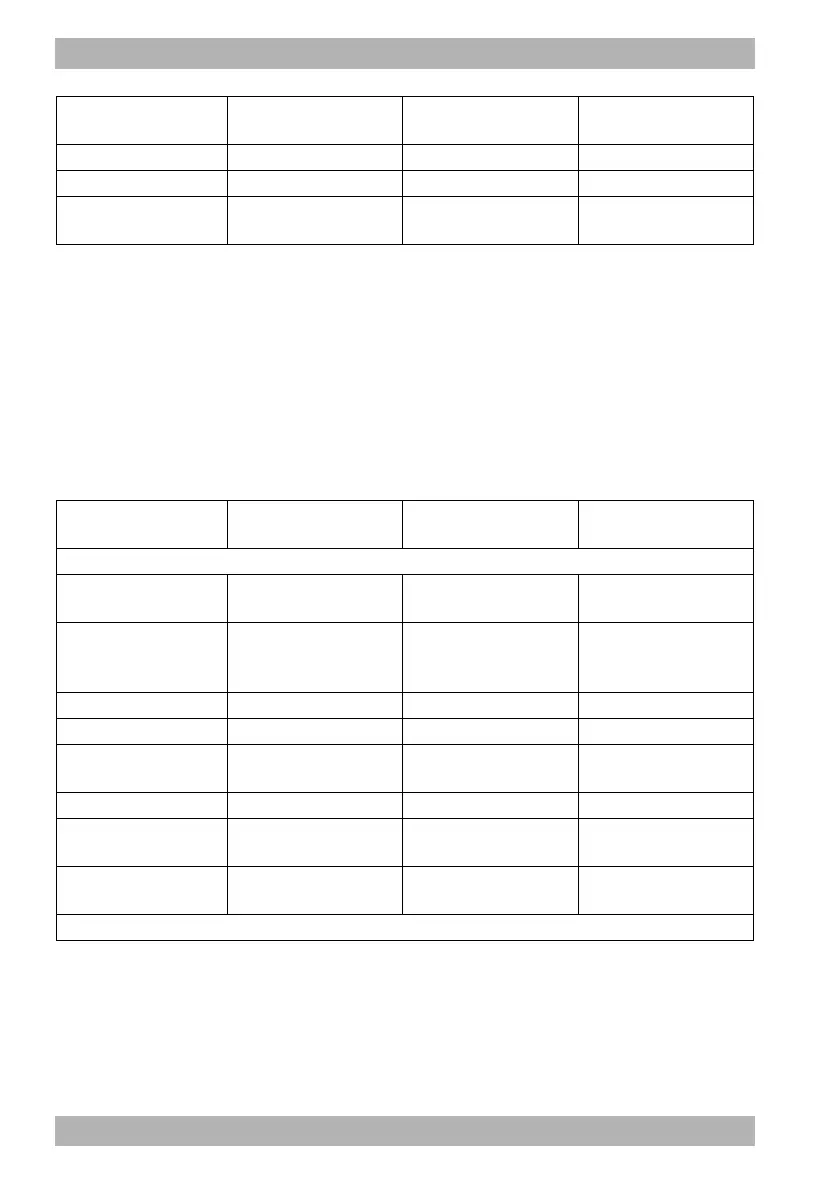
Designation
Supplementary
information
UDI-DI Article no.
Designation
Supplementary
information
UDI-DI Article no.
Options
Manual defibrillation
option
– – WM 45499
Cardioversion option
Requirement: Manual
defibrillation option is
enabled
– WM 45620
Printing option – – WM 45621
12-lead ECG option – – WM 45622
E-mail delivery 12-lead
ECG option
Requirement: 12-lead
ECG option is enabled
– WM 45626
Replay view option – – WM 45628
Bluetooth
®
data
transmission option
– – WM 45624
Upload session data
option
– – WM 45627
 Loading...
Loading...









