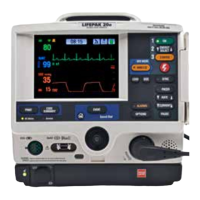
LIFEPAK 15 Monitor/Defibrillator
Service Manual
Introduction
Device Tracking
Section Menu Section Contents Back Index
18
1
Device Tracking
Device Tracking:
The U.S. Food and Drug Administration requires defibrillator manufacturers and distributors to track the location of their defibrillators. If the
device is located somewhere other than the shipping address or the device has been sold, donated, lost, stolen, exported, destroyed, permanently
retired from use, or if the device was not obtained directly from Physio-Control, please do one of the following: register the device at http://
www.physio-control.com, call the device registration phone line at 1.800.426.4448, or use one of the postage-paid address change cards
located in the back of the LIFEPAK 15 Monitor/Defibrillator Operating Instructions, to update this vital tracking information.
 Loading...
Loading...