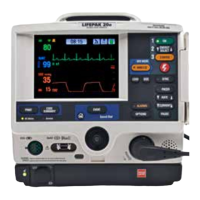
LIFEPAK 15 Monitor/Defibrillator
Service Manual
Assembly Diagrams and Parts Lists
Section Glossary
Section Menu Section Contents
Back
Index
367
9
Section Glossary
The following are definitions of terms used in this section.
• Common parts are components used in every version of the defibrillator device, regardless of options and operating language.
Common parts are divided into Front Case, Rear Case, and System/Therapy PCB Assembly.
• Internal parts are components internal to the case that are specific to your device.
• External parts are components external to the case that are specific to your device.
• The Item number on diagrams (used most often for cables and connectors) provides a reference number for parts on the device.
Click on the item number in a diagram to jump to that part.
• The Quantity column identifies how many of the listed part is used in the assembly.
• MIN refers to the Physio-Control manufacturer’s item number.
• CAT. number is used for ordering each part.
• The Part Description column provides a brief description of each part in the parts list.
• PN is the part number that identifies the model of each LIFEPAK 15 monitor/defibrillator.
• Options are assemblies that are not required on the basic device and can be specified by the customer when purchased. Parts
on these assemblies may be referred to as optional parts.
• V1 (Version 1) refers to LIFEPAK 15 devices that do not have the auxiliary power feature.
• V2 (Version 2) refers to LIFEPAK 15 devices that have the auxiliary power feature. A device must be V2-equivalent to order the
Temperature option.
 Loading...
Loading...