Chapter 5 Ion Measurement (F-53,55)
5.3 Measurement Using Standard Addition Method
72 HORIBA
" ● Types and Features of Applicable Electrodes" page 235
Setting range
For more accurate measurement:
Normally, the added amount of the standard solution should be from 0.1% to 10% volume of
the sample solution. It is desirable that when the standard solution is added to the sample solu
-
tion, the concentration of the ion species under measurement increase by 1 to 10 times in the
sample solution. It is therefore necessary to consider the concentration and added amount of
the standard solution well.If the concentration of the added standard solution is too low, if the
volume of the added standard volume is too larger than that of the sample, then a large error
may occur in the measurement result.
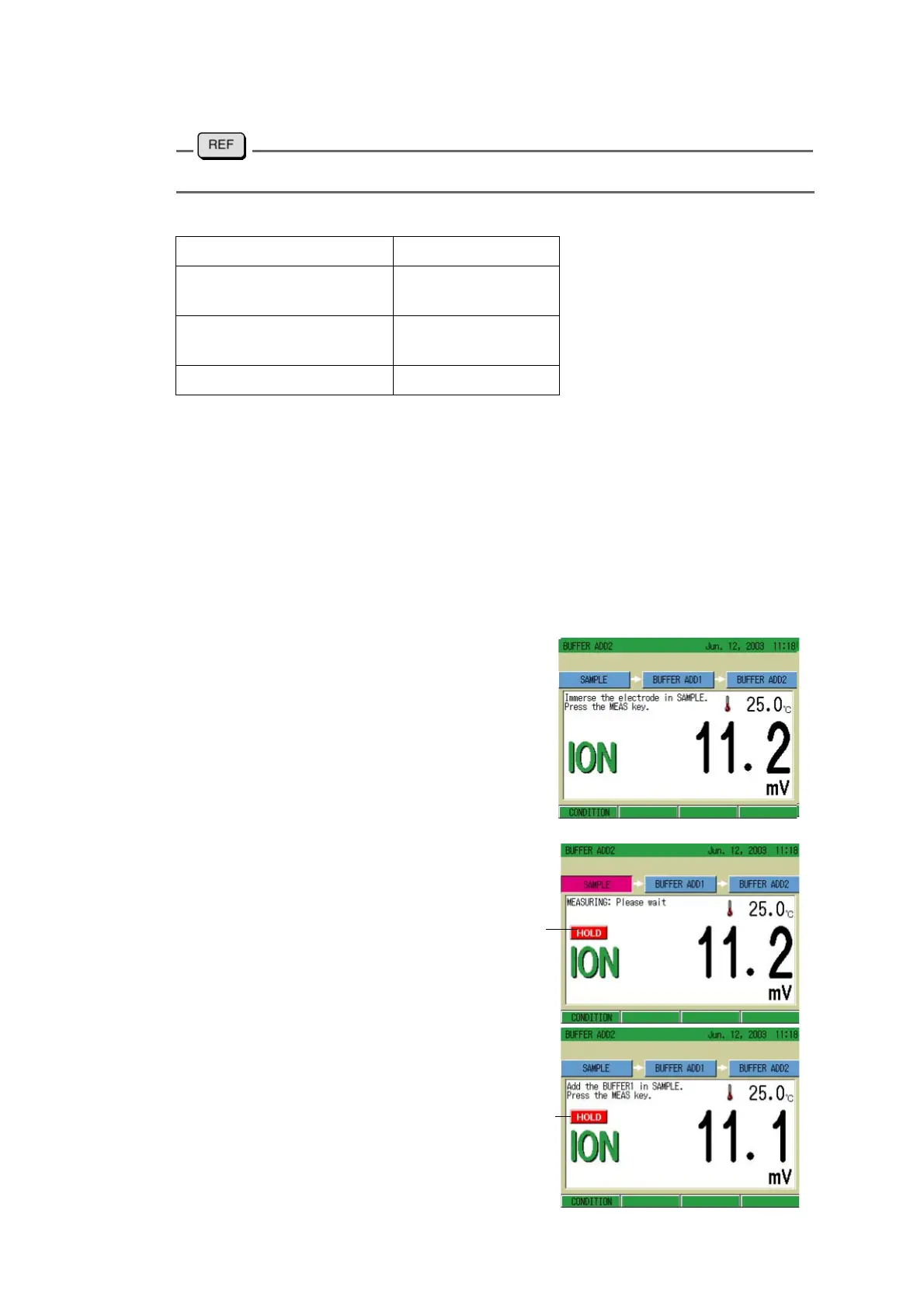
● Measurement
1. Immerse the ion electrode in sam-
ple solution.
2. Press the MEAS key.
The AutoHold measurement starts.
When the indication stabilizes, the
HOLD indication lights up.
Sample volume: 1.0 to 19999.0 mL
Concentration of additive
standard solution:
0.1 to 19000.0 mg/L
Volume 1, 2 of additive
standard solution:
0.01 to 1000 mL
Ion valency: +2, +1, -1, -2
Blinking
Lit
 Loading...
Loading...